Abstract
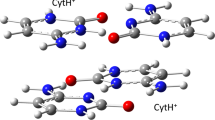
The tricyclic cytosine, tC, is a fluorescent base analogue with excellent properties for investigating intrinsic characteristics of nucleic acid as well as interactions between nucleic acids and other molecules. Its unique fluorescence properties and insignificant influence on overall structure and dynamics of nucleic acid after incorporation makes tC particularly interesting in fluorescence resonance energy transfer and anisotropy measurements. We here describe a straightforward synthesis of the standard monomer form of tC for DNA solid-phase synthesis, the tC phosphoramidite, and its subsequent incorporation into oligonucleotides. The total synthesis of the tC phosphoramidite takes approximately 8 days and its incorporation and the subsequent oligonucleotide purification an additional day.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rist, M.J. & Marino, J.P. Fluorescent nucleotide base analogs as probes of nucleic acid structure, dynamics and interactions. Curr. Org. Chem. 6, 775–793 (2002).
Okamoto, A., Saito, Y. & Saito, I. Design of base-discriminating fluorescent nucleosides. J. Photochem. Photobiol. C 6, 108–122 (2005).
Wilson, J.N. & Kool, E.T. Fluorescent DNA base replacements: reporters and sensors for biological systems. Org. Biomol. Chem. 4, 4265–4274 (2006).
Ward, D.C., Reich, E. & Stryer, L. Fluorescence studies of nucleotides and polynucleotides I. Formycin 2-aminopurine riboside 2,6-diaminopurine riboside and their derivatives. J. Biol. Chem. 244, 1228–1237 (1969).
Holmén, A., Nordén, B. & Albinsson, B. Electronic transition moments of 2-aminopurine. J. Am. Chem. Soc. 119, 3114–3121 (1997).
Hawkins, M.E., Pfleiderer, W., Mazumder, A., Pommier, Y.G. & Falls, F.M. Incorporation of a fluorescent guanosine analog into oligonucleotides and its application to a real-time assay for the Hiv-1 integrase 3′-processing reaction. Nucleic Acids Res. 23, 2872–2880 (1995).
Hawkins, M.E., Pfleiderer, W., Balis, F.M., Porter, D. & Knutson, J.R. Fluorescence properties of pteridine nucleoside analogs as monomers and incorporated into oligonucleotides. Anal. Biochem. 244, 86–95 (1997).
Driscoll, S.L., Hawkins, M.E., Balis, F.M., Pfleiderer, W. & Laws, W.R. Fluorescence properties of a new guanosine analog incorporated into small oligonucleotides. Biophys. J. 73, 3277–3286 (1997).
Hawkins, M.E., Pfleiderer, W., Jungmann, O. & Balis, F.M. Synthesis and fluorescence characterization of pteridine adenosine nucleoside analogs for DNA incorporation. Anal. Biochem. 298, 231–240 (2001).
Hawkins, M.E. Fluorescent pteridine nucleoside analogs—a window on DNA interactions. Cell Biochem. Biophys. 34, 257–281 (2001).
Berry, D.A. et al. Pyrrolo-dC and pyrrolo-C: fluorescent analogs of cytidine and 2′-deoxycytidine for the study of oligonucleotides. Tetrahedron Lett. 45, 2457–2461 (2004).
Guest, C.R., Hochstrasser, R.A., Sowers, L.C. & Millar, D.P. Dynamics of mismatched base-pairs in DNA. Biochemistry 30, 3271–3279 (1991).
Stivers, J.T. 2-Aminopurine fluorescence studies of base stacking interactions at abasic sites in DNA: metal-ion and base sequence effects. Nucleic Acids Res. 26, 3837–3844 (1998).
Hochstrasser, R.A., Carver, T.E., Sowers, L.C. & Millar, D.P. Melting of a DNA helix terminus within the active-site of a DNA-polymerase. Biochemistry 33, 11971–11979 (1994).
Allan, B.W. & Reich, N.O. Targeted base stacking disruption by the EcoRI DNA methyltransferase. Biochemistry 35, 14757–14762 (1996).
Bloom, L.B., Otto, M.R., Beechem, J.M. & Goodman, M.F. Influence of 5′-nearest neighbors on the insertion kinetics of the fluorescent nucleotide analog 2-aminopurine by Klenow fragment. Biochemistry 32, 11247–11258 (1993).
Lycksell, P.O. et al. Base pair opening dynamics of a 2-aminopurine substituted EcoRi restriction sequence and its unsubstituted counterpart in oligonucleotides. Nucleic Acids Res. 15, 9011–9025 (1987).
Stivers, J.T., Pankiewicz, K.W. & Watanabe, K.A. Kinetic mechanism of damage site recognition and uracil flipping by Escherichia coli uracil DNA glycosylase. Biochemistry 38, 952–963 (1999).
Wilhelmsson, L.M., Holmén, A., Lincoln, P., Nielsen, P.E. & Nordén, B. A highly fluorescent DNA base analogue that forms Watson–Crick base pairs with guanine. J. Am. Chem. Soc. 123, 2434–2435 (2001).
Wilhelmsson, L.M. et al. Photophysical characterization of fluorescent DNA base analogue, tC. J. Phys. Chem. B 107, 9094–9101 (2003).
Sandin, P. et al. Fluorescent properties of DNA base analogue tC upon incorporation into DNA––negligible influence of neighbouring bases on fluorescence quantum yield. Nucleic Acids Res. 33, 5019–5025 (2005).
Engman, K.C. et al. DNA adopts normal B-form upon incorporation of highly fluorescent DNA base analogue tC: NMR structure and UV–Vis spectroscopy characterization. Nucleic Acids Res. 32, 5087–5095 (2004).
Kazimierczuk, Z., Cottam, H.B., Revankar, G.R. & Robins, R.K. Synthesis of 2′-deoxytubercidin, 2′-deoxyadenosine, and related 2′-deoxynucleosides via a novel direct stereospecific sodium-salt glycosylation procedure. J. Am. Chem. Soc. 106, 6379–6382 (1984).
Roth, B. & Hitchings, G.H. 5-Arylthiopyrimidines. II. 2- and 4-alkylamino and 4-amino derivatives. J. Org. Chem. 26, 2770–2778 (1961).
Roth, B. & Schloemer, L.A. 5-Arylthiopyrimidines. III. Cyclization of 4-hydroxy derivatives to 10H-pyrimido[5,4-b][1,4]benzothiazines (1,3-diazaphenothiazines). J. Org. Chem. 28, 2659–2672 (1663).
Rolland, V., Kotera, M. & Lhomme, J. Convenient preparation of 2-deoxy-3,5-di-O-p-toluoyl-alpha-D-erythro-pentofuranosyl chloride. Synt. Com. 27, 3505–3511 (1997).
Lin, K.Y., Jones, R.J. & Matteucci, M. Tricyclic 2′-deoxycytidine analogs—syntheses and incorporation into oligodeoxynucleotides which have enhanced binding to complementary RNA. J. Am. Chem. Soc. 117, 3873–3874 (1995).
Brown, T. & Grzybowski, J. Preparation of synthetic oligodeoxynucleotide probes. in Gene Probes: A Practical Approach (Eds. B.D. Hames & S.J. Higgins) Ch. 5 146–167 (IRL Press, Oxford University Press, Oxford, UK, 1995).
Brown, T. & Brown, D.J.S. Purification of synthetic DNA. Methods Enzymol. 211, 20–35 (1992).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sandin, P., Lincoln, P., Brown, T. et al. Synthesis and oligonucleotide incorporation of fluorescent cytosine analogue tC: a promising nucleic acid probe. Nat Protoc 2, 615–623 (2007). https://doi.org/10.1038/nprot.2007.80
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.80
This article is cited by
-
Fluorescent base analogues in gapmers enable stealth labeling of antisense oligonucleotide therapeutics
Scientific Reports (2021)
-
Synthesis, oligonucleotide incorporation and fluorescence properties in DNA of a bicyclic thymine analogue
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.