Abstract
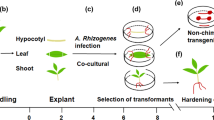
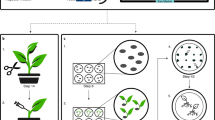
Agrobacterium-mediated transformation is widely used for gene delivery in plants. However, commercial cultivars of crop plants are often recalcitrant to transformation because the protocols established for model varieties are not directly applicable to them. The genus Brassica includes the oil seed crop, canola (B. napus), and vegetable crop varieties of Brassica oleracea, including cauliflower, broccoli and cabbage. Here, we describe an efficient protocol for Agrobacterium-mediated transformation using seedling explants that is applicable to various Brassica varieties; this protocol has been used to genetically engineer commercial cultivars of canola and cauliflower in our laboratory. Young seedling explants are inoculated with Agrobacterium on the day of explant preparation. Explants are grown for 1 week in the absence of a selective agent before being transferred to a selective medium to recover transgenic shoots. Transgenic shoots are subjected to an additional round of selection on medium containing higher levels of the selective agent and a low-carbohydrate source; this helps to eliminate false-positive plants. Use of seedling explants offers flexible experiment planning and a convenient explant source. Using this protocol, transgenic plants can be obtained in 2.5 to 3.5 months.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Òstergaard, L. et al. Pod shatter-resistant Brassica fruit produced by ectopic expression of the FRUITFULL gene. Plant Biotech J. 4, 45–51 (2006).
Cardoza, V. & Stewart, N.C. Canola (Brassica napus L.). in Methods in Molecular Biology Vol. 343 (ed. Wang, K.) 257–265 (Humana Press Inc., Totowa, New Jersey, (2006)).
Poulsen, G.B. Genetic transformation of Brassica. Plant Breed. 115, 209–225 (1996).
Puddephat, I.J. et al. Transformation of Brassica oleracea L: a critical review. Mol. Breed. 2, 185–210 (1996).
Mukhopadhyay, A.R. et al. Efficient regeneration of Brassica oleracea hypocotyl protoplasts and high frequency genetic transformation by direct DNA uptake. Plant Cell Rep. 10, 375–379 (1991).
Radchuk, V.V. et al. Genetic transformation of cauliflower (Brassica oleracea var botrytis) by direct DNA uptake into mesophyll protoplasts. Physiol. Plant. 114, 429–438 (2002).
Eimert, K. & Siegemund, F. Transformation of cauliflower (Brassica oleracea var botrytis): an experimental survey. Plant Mol. Biol. 19, 485–490 (1992).
Bergman, P. & Glimelius, K. Electroporation of rapeseed protoplast—transient and stable transformation. Physiol. Plant. 88, 604–611 (1993).
Herve, C. et al. Molecular analysis of transgenic rapeseed plants obtained by direct gene transfer of two separate plasmids containing, respectively, the cauliflower mosaic virus coat protein gene and a selectable marker gene. Plant Sci. 91, 181–193 (1993).
Jones-Villeneuve, E. et al. Assessment of microinjection for introducing DNA into uninuclear microspores of rapeseed. Plant Cell Tissue Organ Cult. 40, 97–100 (1995).
Chen, J.L. & Beversdorf, W.D. A combined use of microprojectile bombardment and DNA imbibition enhances transformation frequency of canola (Brassica napus L.). Theor. Appl. Genet. 88, 187–192 (1994).
Nehlin, L. et al. Transient β-gus and gfp expression and viability analysis of microprojectile bombarded microspores of Brassica napus L. J. Plant Physiol. 156, 175–183 (2000).
Christey, M.C. & Sinclair, B.K. Regeneration of transgenic kale (Brassica oleracea var acephala), rape (B. napus) and turnip (B. campestris var rapifera) plants via Agrobacterium rhizogenes-mediated transformation. Plant Sci. 87, 161–169 (1992).
Metz, T.D., Dixit, R. & Earle, E.D. Agrobacterium tumefaciens-mediated transformation of broccoli (Brassica oleracea var italica) and cabbage (B. oleracea var capitata). Plant Cell Rep. 15, 287–292 (1995).
Henzi, M.X., Christey, M.C. & McNeil, D.L. Factors that influence Agrobacterium rhizogenes-mediated transformation of broccoli (Brassica oleracea L. var italica). Plant Cell Rep. 19, 994–999 (2000).
Puddephat, I.J. et al. Recovery of phenotypically normal transgenic plants of Brassica oleracea upon Agrobacterium rhizogenes-mediated co-transformation and selection of transformed hairy roots by GUS assay. Mol. Breed. 7, 229–242 (2001).
Oldacres, A.M., Newbury, H.J. & Puddephat, I.J. QTLs controlling the production of transgenic and adventitious roots in Brassica oleracea following treatment with Agrobacterium rhizogenes. Theor. Appl. Genet. 111, 479–488 (2005).
Sparrow, P.A.C. et al. Genetic analysis of Agrobacterium tumefaciens susceptibility in Brassica oleracea. Theor. Appl. Genet. 108, 644–650 (2004).
Sparrow, P.A.C. et al. Genetic analysis of in vitro shoot regeneration from cotyledonary petioles of Brassica oleracea. Theor. Appl. Genet. 108, 1249–1255 (2004).
Rommens, C.M. et al. Crop improvement through modification of the plant's own genome. Plant Physiol. 135, 421–431 (2004).
Nielsen, K.M. Transgenic organisms—time for conceptual diversification. Nat. Biotechnol. 21, 227–228 (2003).
de Block, M., de Brouwer, D. & Tenning, P. Transformation of Brassica napus and Brassica oleracea using Agrobacterium tumefaciens and the expression of bar and neo genes in transgenic plants. Plant Physiol. 91, 694–701 (1989).
Moloney, M.M., Walker, J.M. & Sharma, K.K. High efficiency transformation of Brassica napus using Agrobacterium vectors. Plant Cell Rep. 8, 238–242 (1989).
Barfield, D.G. & Pua, E.C. Gene transfer in plants of Brassica juncea using Agrobacterium tumefaciens-mediated transformation. Plant Cell Rep. 10, 308–314 (1991).
Radke, S.E., Turner, J.C. & Facciotti, D. Transformation and regeneration of Brassica rapa using Agrobacterium tumefaciens. Plant Cell Rep. 11, 499–505 (1992).
Gupta, V. et al. Genetic transformation of Brassica nigra by Agrobacterium-based vector and direct plasmid uptake. Plant Cell Rep. 12, 418–421 (1993).
Damgaard, O. et al. Agrobacterium tumefaciens-mediated transformation of Brassica napus winter cultivars. Transgenic Res. 6, 279–288 (1997).
Wang, W.C. et al. Development of a novel Agrobacterium-mediated transformation method to recover transgenic Brassica napus plants. Plant Cell Rep. 22, 274–281 (2003).
Kazan, K., Curtis, M.D., Goulter, K.C. & Manners, J.M. Agrobacterium tumefaciens-mediated transformation of double haploid canola (Brassica napus) lines. Aust. J. Plant Physiol. 24, 97–102 (1997).
Stewart, C.N. et al. Insect control and dosage effects in transgenic canola containing a synthetic Bacillus thuringiensis crylAc gene. Plant Physiol. 112, 115–120 (1996).
Zhang, Y., Singh, M.B., Swoboda, I. & Bhalla, P.L. Agrobacterium-mediated transformation and generation of male sterile lines of Australian canola. Aust. J. Agric. Res. 56, 353–361 (2005).
Bhalla, P.L. & Smith, N. Agrobacterium-mediated transformation of Australian cultivars of cauliflowers, Brassica oleracea var botrytis. Mol. Breed. 4, 531–541 (1998).
Bhalla, P.L. & Smith, N. Comparison of regeneration potential from seedling explants of Australian cauliflower (Brassica oleracea) varieties. Aust. J. Agric. Res. 49, 1261–1266 (1998).
Zhang, Y. & Bhalla, P.L In vitro shoot regeneration from commercial cultivars of Australian canola (Brassica napus L.). Aust. J. Agric. Res. 55, 753–756 (2004).
Murashige, T. & Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497 (1962).
Acknowledgements
We acknowledge the financial support from the Australian Research Council (ARC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhalla, P., Singh, M. Agrobacterium-mediated transformation of Brassica napus and Brassica oleracea. Nat Protoc 3, 181–189 (2008). https://doi.org/10.1038/nprot.2007.527
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.527
This article is cited by
-
Genetic manipulation of Indian mustard genotypes with WRR-gene(s) confers resistance against Albugo candida
Molecular Biology Reports (2024)
-
A natural mutation in the promoter of Ms-cd1 causes dominant male sterility in Brassica oleracea
Nature Communications (2023)
-
Generation of stable transgenic Brassica napus cv. Jet Neuf cell cultures as a tool to investigate in planta protein function
Plant Cell, Tissue and Organ Culture (PCTOC) (2023)
-
Establishing a highly efficient Agrobacterium-mediated transformation system in sweet buckwheat
Journal of Consumer Protection and Food Safety (2023)
-
Sef1, rapid-cycling Brassica napus for large-scale functional genome research in a controlled environment
Theoretical and Applied Genetics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.