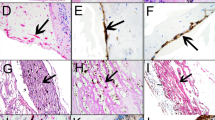
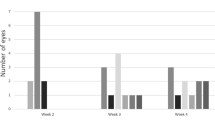
Abstract
We outline current in vitro and in vivo models for experimental proliferative vitreoretinopathy (PVR) and provide a detailed protocol of our standardized in vivo PVR model. PVR is the leading cause of failed surgical procedures for the correction of rhegmatogenous retinal detachment. The pathogenesis of this multifactorial condition is still not completely understood. Experimental models for PVR help us understand the factors that play a role in the pathogenesis of the disease process in a controlled manner and allow for reproducible preclinical assessment of novel therapeutic interventions. We describe a cell injection model in detail that uses homologous retinal pigment epithelial (RPE) cell cultures to induce PVR over a 2–8 week period.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Retina Society Terminology Committee. The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology 90, 121–125 (1983).
Machemer, R. Pathogenesis and classification of massive periretinal proliferation. Br. J. Ophthalmol. 62, 737–747 (1978).
Silicone Study Group. Proliferative vitreoretinopathy (Editorial). Am. J. Ophthalmol. 99, 593–595 (1985).
Green, W.G. & Sebag, J. Vitreoretinal interface. in Retina 4th edn. Vol. 3 (ed. Ryan, S.J.) 1921–1989 (Elsevier Mosby, St. Louis, 2006).
Thompson, J.T. Proliferative vitreoretinopathy. in Retina 4th edn. Vol. 3 (ed. Ryan, S.J.) 2282–2309 (Elsevier Mosby, St. Louis, 2006).
Vinores, S.A., Campochiaro, P.A. & Conway, B.P. Ultrastructural and electron-immunocytochemical characterization of cells in epiretinal membranes. Invest. Ophthalmol. Vis. Sci. 31, 14–28 (1990).
Charteris, D.G. Proliferative vitreoretinopathy: Pathobiology, surgical management, and adjunctive treatment. Br. J. Ophthalmol. 79, 953–960 (1995).
Weller, M., Esser, P., Bresgen, M., Heimann, K. & Wiedemann, P. Thrombospondin: a new attachment protein in preretinal traction membranes. Eur. J. Ophthalmol. 2, 10–14 (1992).
Hiscott, P.S., Grierson, I. & McLeod, D. Natural history of fibrocellular epiretinal membranes: a quantitative, autoradiographic, and immunohistochemical study. Br. J. Ophthalmol. 11, 810–823 (1985).
Cordeiro, M.F. et al. Molecular therapy in ocular wound healing. Br. J. Ophthalmol. 83, 1219–1224 (1999).
Campochiaro, P.A. Pathogenesis of proliferative vitreoretinopathy. in Retina 3rd edn. Vol. 3 (ed. Ryan, S.J.) 2221–2227 (Mosby, St. Louis, 2001).
Thumann, G. & Hinton, D.R. Cell biology of the retinal pigment epithelium. in Retina 3rd edn. Vol. 1 (ed. Ryan, S.J.) 104–121 (Mosby, St. Louis, 2001).
Anderson, D.H., Stern, W.H., Fisher, S.K., Erickson, P.A. & Borgula, G.A. The onset of pigment epithelial proliferation after retinal detachment. Invest. Ophthalmol. Vis. Sci. 21, 10–16 (1981).
Jin, M., He, S., Worpel, V., Ryan, S.J. & Hinton, D.R. Promotion of adhesion and migration of RPE cells to provisional extracellular matrices by TNF-α. Invest. Ophthalmol. Vis. Sci. 41, 4324–4332 (2000).
Hinton, D.R. et al. Mitogen-activated protein kinase activation mediates PDGF-directed migration of RPE cells. Exp. Cell Res. 239, 11–15 (1998).
Limb, G.A., Hollifield, R.D., Webster, L., Charteris, D.G. & Chignell, A.H. Soluble TNF receptors in vitreoretinal proliferative disease. Invest. Opthalmol. Vis. Sci. 42, 1586–1591 (2001).
Campochiaro, P.A., Sugg, R., Grotendorst, G. & Hjelmeland, L.M. Retinal pigment epithelial cells produce PDGF-like proteins and secrete them into their media. Exp. Eye Res. 49, 217–227 (1989).
Andrews, A. et al. Platelet-derived growth factor plays a key role in proliferative vitreoretinopathy. Invest. Ophthalmol. Vis. Sci. 40, 2683–2689 (1999).
Ikuno, Y., Leong, F.L. & Kazlauskas, A. Attenuation of experimental proliferative vitreoretinopathy by inhibiting the platelet-derived growth factor receptor. Invest. Ophthalmol. Vis. Sci. 41, 3107–3116 (2000).
Seo, M.S. et al. Photoreceptor-specific expression of platelet-derived growth factor-B results in traction retinal detachment. Am. J. Pathol. 157, 995–1005 (2000).
Guidry, C. Tractional force generation by porcine muller cells development & differential stimulation by growth factors. Invest. Ophthalmol. Vis. Sci. 38, 456–468 (1997).
Hardwick, C. et al. Tractional force generation by porcine muller cells stimulation by growth factors in human vitreous. Invest. Ophthalmol. Vis. Sci. 38, 2053–2063 (1997).
Pfeffer, B.A., Flanders, K.C., Guerin, C.J., Danielpour, D. & Anderson, D.H. Transforming growth factor beta 2 is the predominant isoform in the neural retina, retinal pigment epithelium-choroid and vitreous of the monkey eye. Exp. Eye. Res. 59, 323–333 (1994).
Connor, T.B. Jr. et al. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J. Clin. Invest. 83, 1661–1666 (1989).
Bornstein, P. Thrombospondins as matricellular modulators of cell function. J. Clin. Invest. 107, 929–934 (2001).
Koli, K., Saharinen, J., Hyytiainen, M., Penttinen, C. & Keski-Oja, J. Latency, activation, and binding proteins of TGF-beta. Microsc. Res. Tech. 52, 354–362 (2001).
Hinton, D.R., He, S., Jin, M.L., Barron, E. & Ryan, S.J. Novel growth factors involved in the pathogenesis of proliferative vitreoretinopathy. Eye 16, 422–428 (2002).
Forrester, J.V., Docherty, R., Kerr, C. & Lackief, J.M. Cellular proliferation in the vitreous: the use of vitreous explants as a model system. Invest. Ophthalmol. Vis. Sci. 27, 1085–1094 (1986).
Gamulescu, M.A. et al. Transforming growth factor beta-2 induced myofibroblastic differentiation of human pigment epithelial cells: regulation by extracellular matrix protein and hepatocyte growth factor. Exp Eye Res. 83, 212–222 (2006).
Blumenkranz, M.S. & Hartzer, M.K. The mechanism of action of drugs for the treatment of vitreoretinal scarring. in Retina 2nd edn. Vol. 3 (ed. Ryan, S.J.) 2281–2300 (CV Mosby, St. Louis, 1994).
Kimura, H. et al. Retrovirus-mediated suicide gene transduction in the vitreous cavity of the eye: Feasibility in prevention of proliferative vitreoretinopathy. Hum. Gene Ther. 7, 799–808 (1996).
Lean, J.S., van der Zee, W.A.M. & Ryan, S.J. Experimental model of proliferative vitreoretinopathy (PVR) in the vitrectomized eye: effect of silicone oil. Br. J. Ophthalmol 68, 332–335 (1984).
Allamby, D., Foreman, D., Carrington, L., McLeod, D. & Boulton, M. Cell attachment to, and contraction of, the retina in vitro . Invest. Ophthalmol. Vis. Sci. 38, 2064–2072 (1997).
Luo, J. & Miller, M.W. Platelet-derived growth factor-mediated signal transduction underlying astrocyte proliferation: site of ethanol action. J. Neurosci. 19, 10014–10125 (1999).
Fujisawa, K., Spee, C., Hinton, D.R. & Ryan, S.J. PDGF-BB stimulates gliosis in a mouse organ culture model of PVR. Invest. Ophthalmol. Vis. Sci. 44 ARVO E-Abstract 4982 (2003).
de Souza, O.F. et al. Inhibition of experimental proliferative vitreoretinopathy in rabbits by suramin. Ophthalmologica 209, 212–216 (1995).
Fastenberg, D.M., Diddie, K.R., Sorgente, N. & Ryan, S.J. A comparison of different cellular innocula in an experimental model of massive periretinal proliferation. Am. J. Ophthalmol. 93, 559–564 (1982).
Fastenberg, D.M., Diddie, K.R., Dorey, K. & Ryan, S.J. The role of cellular proliferation in an experimental model of massive periretinal proliferation. Am. J. Ophthalmol. 93, 565–572 (1982).
Wiedermann, P., Sorgente, S. & Ryan, S. Proliferative vitreoretinopathy: The rabbit cell injection model for screening of antiproliferative drugs. J. Pharmacol. Methods 12, 69–78 (1984).
Tano, Y., Sigita, G., Abrams, G. & Machemer, R. Inhibition of intraocular proliferation with intravitreal proliferations with intravitreal corticosteroids. Am. J. Ophthalmol. 89, 131–136 (1980).
Hida, T., Chandler, D.B. & Sheta, S.M. Classification of the stages of proliferative vitreoretinopathy in a refined experimental model in the rabbit eye. Graefes Arch. Clin. Exp. Ophthalmol. 225, 303–307 (1987).
Hsu, H.T., Dorey, K., Sorgente, N. & Ryan, S.J. Surgical removal of vitreous. Its effect on intraocular fibroblast proliferation in the vitreous. Arch. Ophthalmol. 102, 605–607 (1984).
Sugita, G. et al. Intravitreal autotransplantation of fibroblasts. Am. J. Ophthalmol. 89, 121–130 (1980).
Radtke, N.D., Tano, Y., Chandler, D. & Machemer, R. Simulation of massive periretinal proliferation by autotransplantation of retinal pigment cells in rabbits. Am. J. Ophthalmol. 91, 76–87 (1981).
Yeo, J.H., Sadeghi, J., Campochiaro, P.A., Green, W.R. & Glaser, B.M. Intravitreous fibronectin and platelet-derived growth factor. Arch. Ophthalmol. 104, 417–421 (1986).
Cleary, P.E. & Ryan, S.J. Method of production and natural history of experimental posterior penetrating eye injury in the rhesus monkey. Am. J. Ophthalmol. 88, 212–220 (1979).
Wilson, C.A., Khawly, J.A., Hatchell, D.L. & Machemer, R. Experimental traction retinal detachment in the cat. Graefes Arch. Clin. Exp. Ophthalmol. 229, 568–573 (1991).
Valeria Canto, S.M., Gallo, J.E., Dodds, R.A. & Suburo, A.M. A mouse model of proliferative vitreoretinopathy induced by dispase. Exp. Eye. Res. 75, 491–504 (2002).
Vergara, O., Ogden, T. & Ryan, S.J. Posterior penetrating injury to the rabbit eye: Effect of blood and ferrous ions. Exp. Eye. Res. 49, 1115–1126 (1989).
Cardillo, J.A. et al. Post-traumatic proliferative vitreoretinopathy: The epidemiologic profile, onset, risk factors and visual outcome. Ophthalmology 104, 1166–1173 (1997).
Hsu, H.T. & Ryan, S.J. Natural history of penetrating ocular injury with retinal laceration in the monkey. Graefes Arch. Clin. Exp. Ophthalmol. 224, 1–6 (1986).
Cleary, P.E. & Ryan, S.J. Experimental posterior penetrating eye injury in the rabbit: 1. Method of production and natural history. Br. J. Ophthalmol. 63, 306–311 (1979).
Cleary, P.E. & Ryan, S.J. Experimental posterior penetrating eye injury in the rabbit. II. Histology of wound, vitreous, and retina. Br. J. Ophthalmol. 63, 312–321 (1979).
Cleary, P.E. & Ryan, S.J. Vitrectomy in penetrating eye injury. Results of a controlled trial of vitrectomy in an experimental posterior penetrating eye injury in the rhesus monkey. Arch. Ophthalmol. 99, 287–292 (1981).
Kimura, H. et al. Cellular response in subretinal neovascularization induced by bFGF-impregnated microspheres. Invest. Ophthalmol. Vis. Sci. 40, 524–528 (1999).
Latendresse, J.R., Warbrittion, A.R., Jonassen, H. & Creasy, D.M. Fixation of testes and eyes using a modified Davidson's fluid: comparison with Bouin's fluid and conventional Davidson's fluid. Toxicol. Pathol. 30, 524–533 (2002).
Algvere, P. & Kock, E. Experimental fibroplasia in the rabbit vitreous. Retinal detachment induced by autologous fibroblasts. Albrecht von Graefes Arch. Klin. Exp. Ophthalmol. 199, 215–222 (1976).
Gonvers, M. & Thrasher, R. Temporary use of silicone oil in the treatment of proliferative vitreoretinopathy: an experimental study with a new animal model. Graefes Arch. Clin. Exp. Ophthalmol. 221, 46–53 (1983).
Chandler, D.B., Quansah, F.A., Hida, T. & Machemer, R. A refined experimental model for proliferative vitreoretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 224, 86–91 (1986).
Sakamoto, T. et al. Inhibition of experimental proliferative vitreoretinopathy by retroviral vector-mediated transfer of suicide gene. Ophthalmology 102, 1417–1424 (1995).
Wong, C.A. et al. Induction of proliferative vitreoretinopathy by a unique line of human retinal pigment epithelial cells. Can. J. Ophthalmol. 37, 211–220 (2002).
Hui, Y.N., Sorgente, N. & Ryan, S.J. Posterior vitreous separation and retinal detachment induced by macrophages. Graefes Arch. Clin. Exp. Ophthalmol. 225, 279–284 (1987).
Hui, Y.N., Goodnight, R., Sorgente, N. & Ryan, S.J. Fibrovascular proliferation and retinal detachment after intravitreal injection of activated macrophages in the rabbit eye. Am. J. Ophthalmol. 108, 176–184 (1989).
Nakagawa, M., Refojo, M.F., Marin, J.F., Doi, M. & Tolentino, F.I. Retinoic acid in silicone and silicone-fluorosilicone copolymer oils in a rabbit model of proliferative vitreoretinopathy. Invest. Ophthalmol. Vis. Sci. 36, 2388–2395 (1995).
Pinon, R.M. et al. Intravitreal and subretinal proliferation induced by platelet-rich plasma injection in rabbits. Curr. Eye Res. 11, 1047–1055 (1992).
Goldaracena, M.B. et al. The role of retinotomy in an experimental rabbit model of proliferative vitreoretinopathy. Curr. Eye Res. 16, 422–427 (1997).
Pastor, J.C., Rodriguez, E., Marcos, M.A. & Lopez, M.I. Combined pharmacologic therapy in a rabbit model of proliferative vitreoretinopathy. Ophthalmic Res. 32, 25–29 (2000).
Garcia-Layana, A., Pastor, J.C., Saornil, M.A. & Gonzalez, G. Porcine model of proliferative vitreoretinopathy with platelets. Curr. Eye. Res. 16, 556–563 (1997).
Chinn, C., Spee, C., Barron, E., Ryan, S.J. & Hinton, D.R. Strain-dependent gene expression in a lens extraction PVR model. Invest. Ophthlamol. Vis. Sci. 46 ARVO E-Abstract 5528 (2005).
Saika, S. et al. Inhibition of p38MAP kinase suppresses fibrotic reaction of retinal pigment epithelial cells. Lab. Invest. 85, 838–850 (2005).
Saika, S. et al. Smad3 is required for dedifferentiation of retinal pigment epithelium following retinal detachment in mice. Lab. Invest. 84, 1245–1258 (2004).
Campochiaro, P.A., Gaskin, H.C. & Vinores, S.A. Retinal cryopexy stimulates traction retinal detachment formation in the presence of an ocular wound. Arch. Ophthalmol. 105, 1567–1570 (1987).
Liou, G.I. et al. HGF regulation of RPE proliferation in an IL-1 beta/retinal hole-induced rabbit model of PVR. Mol. Vis. 8, 494–501 (2002).
Planck, S.R. et al. Expression of growth factor mRNA in rabbit PVR model systems. Curr. Eye. Res. 11, 1031–1039 (1992).
Frenzel, E.M., Neely, K.A., Walsh, A.W., Cameron, J.D. & Gregerson, D.S. A new model of proliferative vitreoretinopathy. Invest. Ophthalmol. Vis. Sci. 39, 2157–2164 (1998).
Kralinger, M.T. et al. Experimental model for proliferative vitreoretinopathy by intravitreal dispase: limited by zonulysis and cataract. Ophthalmologica 220, 211–216 (2006).
Yamada, H. et al. Platelet-derived growth factor-A-induced retinal gliosis protects against ischemic retinopathy. Am. J. Pathol. 156, 477–487 (2000).
Campochiaro, P.A., Hackett, S.F. & Vinores, S.A. Growth factors in the retina and retinal pigmented epithelium. Prog. Retinal Eye Res. 15, 547–567 (1996).
Akiyama, H. et al. Intraocular injection of an aptamer that binds PDGF-B: A potential treatment for proliferative retinopathies. J. Cell Physiol. 207, 407–412 (2006).
Jin, M., Chen, Y., He, S., Ryan, S.J. & Hinton, D.R. Hepatocyte growth factor and its role in the pathogenesis of retinal detachment. Invest. Ophthalmol. Vis. Sci. 45, 323–329 (2004).
Blumenkranz, M.S., Ophir, A., Claflin, A.J. & Kajek, A. Fluorouracil for the treatment of massive preretinal proliferation. Am. J. Ophthalmol. 94, 458–467 (1982).
Acknowledgements
The authors would like to thank Ernesto Barron for assistance with the figures and Susan Clarke for editorial assistance. This work was supported by National Institutes of Health grant EY02061 and a National Eye Institute core grant to the Doheny Eye Institute (EY03040).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Agrawal, R., He, S., Spee, C. et al. In vivo models of proliferative vitreoretinopathy. Nat Protoc 2, 67–77 (2007). https://doi.org/10.1038/nprot.2007.4
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.4
This article is cited by
-
A simulacrum of proliferative vitreoretinopathy (PVR): development and proteomics-based validation of an in vitro model
Journal of Proteins and Proteomics (2024)
-
Injectable in situ cross-linking hyaluronan hydrogel for easier removal of posterior vitreous cortex in vitrectomy
Japanese Journal of Ophthalmology (2024)
-
Ligand-independent activation of platelet-derived growth factor receptor β promotes vitreous-induced contraction of retinal pigment epithelial cells
BMC Ophthalmology (2023)
-
Intravitreal allogeneic mesenchymal stem cells: a non-randomized phase II clinical trial for acute non-arteritic optic neuropathy
Stem Cell Research & Therapy (2023)
-
DAPL1 prevents epithelial–mesenchymal transition in the retinal pigment epithelium and experimental proliferative vitreoretinopathy
Cell Death & Disease (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.