Abstract
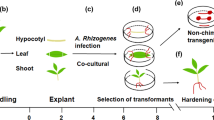
This protocol is used to induce transgenic roots on soybean to study the function of genes required in biological processes of the root. Young seedlings with unfolded cotyledons are infected at the cotyledonary node and/or hypocotyl with Agrobacterium rhizogenes carrying the gene construct to be tested and the infection sites are kept in an environment of high humidity. When the emerged hairy roots can support the plants, the main roots are removed and the transgenic roots can be tested. Using this method, almost 100% of the infected plants form hairy roots within 1 month from the start of the experiments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Graham, P.H. & Vance, C.P. Legumes: importance and constraints to greater use. Plant Physiol. 131, 872–877 (2003).
Kinkema, M., Scott, P.T. & Gresshoff, P.M. Legume nodulation: successful symbiosis through short- and long-distance signalling. Funct. Plant Biol. 33, 707–721 (2006).
Ash, M. Oilseeds. Agricultural Outlook 238, 4 (1997).
Brar, G.S. & Carter, T.E. Soybean Glycine max (L.) Merrill. in Genetic Improvement of Vegetable Crops (eds. Kalloo, G. & Bergh, B.O.) 427–463 (Pergamon Press, New York, USA, 1993).
Song, Q.J. et al. A new integrated genetic linkage map of the soybean. Theor. Appl. Genet. 109, 122–128 (2004).
Shoemaker, R. et al. A compilation of soybean ESTs: generation and analysis. Genome 45, 329–338 (2002).
Maguire, T.L. et al. Tissue-specific gene expression in soybean (Glycine max) detected by cDNA microarray analysis. J. Plant Physiol. 159, 1361–1374 (2002).
Finer, J.J. & McMullen, M.D. Transformation of soybean via particle bombardment of embryogenic suspension culture tissue. In Vitro Cell Dev. Biol. 27P, 175–182 (1991).
Hinchee, M.A.W. et al. Production of transgenic soybean plants using Agrobacterium-mediated DNA transfer. Bio/Technology 6, 915–922 (1988).
McCabe, D.E., Swain, W.F., Martinell, B.J. & Christou, P. Stable transformation of soybean (Glycine max) by particle acceleration. Bio/Technology 6, 923–926 (1988).
Parrott, W.A., Hoffman, L.M., Hildebrand, D.F., Williams, E.G. & Collins, G.B. Recovery of primary transformants of soybean. Plant Cell Rep. 7, 615–617 (1989).
Trick, H.N. & Finer, J.J. Sonication-assisted Agrobacterium-mediated transformation of soybean [(Glycine max (L.) Merrill] embryogenic suspension culture tissue. Plant Cell Rep. 17, 482–488 (1998).
Chilton, M.D. et al. Agrobacterium rhizogenes inserts T-DNA into the genome of the host plant root cells. Nature 295, 432–434 (1982).
Savka, M.A., Ravillion, B., Noel, G.R. & Farrand, S.K. Induction of hairy roots on cultivated soybean genotypes and their use to propagate the soybean cyst nematode. Phytopathology 80, 503–508 (1990).
Collier, R., Fuchs, B., Walter, N., Lutke, W.K. & Taylor, C.G. Ex vitro composite plants: an inexpensive, rapid method for root biology. Plant J. 43, 449–457 (2005).
Cheon, C.-I., Lee, N., Siddique, A., Bal, A. & Verma, D.P. Roles of plant homologues of Rab1p and Rab7p in the bio-genesis of the peribacteroid membrane, a subcellular compartment formed de novo during root nodule symbiosis. EMBO J. 12, 4125–4135 (1993).
Estrada-Navarrete, G. et al. Agrobacterium rhizogenes transformation of the Phaseolus spp.: a tool for functional genomics. Mol. Plant–Microbe Interact. 19, 1385–1393 (2006).
Beach, K. & Gresshoff, P.M. Characterization and culture of Agrobacterium rhizogenes transformed roots of forage legumes. Plant Sci. 57, 73–81 (1988).
Stiller, J. et al. High frequency transformation and regeneration of transgenic plants in the model legume Lotus japonicus . J. Exp. Bot. 48, 1357–1365 (1997).
Vieweg, M.F. et al. The promoter of the Vicia faba L. gene VfLb29 is specifically activated in the infected cells of root nodules and in the arbuscule-containing cells of mycorrhizal roots from different legume and non-legume plants. Mol. Plant–Microbe Interact. 17, 62–69 (2004).
Broughton, W.J. & Dilworth, M.J. Control of leghemoglobin synthesis in snake beans. Biochem. J. 125, 1075–1080 (1971).
Kouchi, H. et al. Rice ENOD40: isolation and expression analysis in rice and transgenic soybean root nodules. Plant J. 18, 121–129 (1999).
Acknowledgements
We thank the Australian Research Council for an ARC Centre of Excellence grant (CEO348212), the University of Queensland Strategic Research Fund and the Queensland Government Smart State Initiative for funding. A.I. and S.N. acknowledge the PhD scholarship from AUSAID and from the Institute for the Promotion of Teaching Science and Technology (IPST), Thailand, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Note
Selected publications on hairy root transformation of leguminous plants (PDF 79 kb)
Rights and permissions
About this article
Cite this article
Kereszt, A., Li, D., Indrasumunar, A. et al. Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nat Protoc 2, 948–952 (2007). https://doi.org/10.1038/nprot.2007.141
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.141
This article is cited by
-
Agrobacterium rhizogenes-mediated marker-free transformation and gene editing system revealed that AeCBL3 mediates the formation of calcium oxalate crystal in kiwifruit
Molecular Horticulture (2024)
-
The mechanism of low blue light-induced leaf senescence mediated by GmCRY1s in soybean
Nature Communications (2024)
-
Identification of GmPT proteins and investigation of their expressions in response to abiotic stress in soybean
Planta (2024)
-
An ex vitro hairy root system from petioles of detached soybean leaves for in planta screening of target genes and CRISPR strategies associated with nematode bioassays
Planta (2024)
-
Highly efficient Agrobacterium rhizogenes-mediated transformation for functional analysis in woodland strawberry
Plant Methods (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.