Abstract
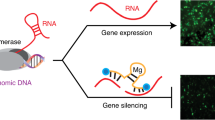
The value of recognizing cellular RNA sequences by short interfering RNAs (siRNAs) in mammalian cells is widely appreciated, but what might be learned if it were also possible to recognize chromosomal DNA? Recognition of chromosomal DNA would have many applications, such as inhibiting gene expression, activating gene expression, introducing mutations, and probing chromosome structure and function. We have shown that antigene peptide nucleic acids (agPNAs) and antigene duplex RNAs (agRNAs) block gene expression and probe chromosomal DNA. Here we describe a protocol for designing antigene agents and introducing them into cells. This protocol can also be used to silence expression with PNAs or siRNAs that target mRNA. From preparation of oligomers to analysis of data, experiments with agPNAs and agRNAs require ∼14 d and 9 d, respectively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kaihatsu, K., Janowski, B.A. & Corey, D.R. Recognition of duplex DNA by oligonucleotides and peptide nucleic acids. Chem. Biol. 11, 749–758 (2004).
Janowski, B.A. et al. Inhibiting transcription of chromosomal DNA using antigene peptide nucleic acids. Nat. Chem. Biol. 1, 210–215 (2005).
Janowski, B.A. et al. Inhibiting gene expression at transcription start sites in chromosomal DNA by antigene RNAs. Nat. Chem. Biol. 1, 216–222 (2005).
Knauert, M.P. & Glazer, P.M. Triplex forming oligonucleotides: sequence-specific tools for gene targeting. Hum. Mol. Gen. 10, 2243–2251 (2001).
Besch, R., Giovannangeli, C., Schuh, T., Kammerbauer, C. & Degitz, K. Characterization and quantification of triple helix formation in chromosomal DNA. J. Mol. Biol. 341, 979–989 (2004).
Dervan, P.B. & Edelson, B.S. Recognition of the DNA minor groove by pyrrole-imidizole polyamides. Curr. Opin. Struct. Biol. 13, 284–299 (2003).
Dudouet, B. et al. Accessibility of nuclear chromatin by DNA binding polyamides. Chem. Biol. 10, 859–867 (2003).
Nielsen, P.G., Egholm, M., Berg, R.H. & Buchardt, O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 254, 1497–1500 (1991).
Hamilton, S.E., Simmons, C.G., Kathriya, I. & Corey, D.R. Cellular delivery of peptide nucleic acids and inhibition of human telomerase. Chem. Biol. 6, 343–351 (1999).
Doyle, D.F., Braasch, D.A, Simmons, C.G., Janowski, B.A. & Corey, DR. Intracellular delivery and inhibition of gene expression by peptide nucleic acids. Biochemistry 40, 53–64 (2001).
Kaihatsu, K., Huffman, K.E. & Corey, D.R. Cellular uptake, localization, and inhibition of gene expression by PNAs and PNA-peptide conjugates. Biochemistry 43, 14340–14347 (2004).
Turner, J.J. et al. Cell-penetrating peptide conjugates of peptide nucleic acids (PNA) as inhibitors of HIV-1 Tat-dependent trans-activation in cells. Nucleic Acids Res. 30, 6837–6849 (2005).
Albertshofer, K. et al. Structure-activity relationship study on a simple cationic peptide motif for cellular delivery of antisense peptide nucleic acid. J. Med. Chem. 48, 6741–6749 (2005).
Maier, M.A. et.al. Evaluation of basic amphipathic peptides for cellular delivery of antisense peptide nucleic acids. J. Med. Chem. 49, 2534–2542 (2006).
Shiraishi, T. et al. Calcium ions effectively enhance the effect of antisense peptide nucleic acids conjugated to cationic tat and oligoarginine peptides. Chem. Biol. 12, 923–929 (2005).
Abes, S. et al. Endosome trapping limits the efficiency of splicing correction by PNA-oligolysine conjugates. J. Control. Release 110, 595–604 (2006).
Nielsen, P.E. Addressing the challenges of cellular delivery and bioavailability of peptide nucleic acids (PNA). Q. Rev. Biophys. 39, 1–6 (2006).
Morris, K.V., Chan, S.W., Jacobsen, S.E. & Looney, D.J. Small interfering RNA-induced transcriptional silencing in human cells. Science 305, 1289–1292 (2004).
Ting, A.H. et al. Short double-stranded RNA induces transcriptional gene silencing in human cells in the absence of DNA methylation. Nat. Genet. 37, 906–910 (2005).
Castanotto, D. et al. Short hairpin RNA-directed cytosine (CpG) methylation of the RASSF1 promoter in HeLa cells. Mol. Ther. 12, 179–183 (2005).
Suzuki, K. et al. Prolonged transcriptional silencing and CpG methylation induced by siRNAs targeted to the HIV-1 promoter region. J. RNAi Gene Silencing 1, 66–78 (2005).
Zhang, M.-X. et al. Regulation of endothelial nitric oxide synthase by small RNA. Proc. Natl. Acad. Sci. USA 102, 16967–16972 (2005).
Weinberg, M.S. et al. The antisense strand of small interfering RNAs directs histone methylation and transcriptional gene silencing in human cells. RNA 12, 256–262 (2006).
Corey, D.R. Regulating mammalian transcription with RNA. Trends Biochem. Sci. 30, 655–658 (2005).
Morris, K.V. Therapeutic potential of siRNA-mediated transcriptional gene silencing. Biotechniques 40, S7–S13 (2006).
Greenberg, M. in Current Protocols in Molecular Biology 4, 10.1–4.10.11 (1987).
Braasch, D.A. & Corey, D.R. Synthesis, analysis, purification, and intracellular delivery of peptide nucleic acids. Methods 23, 97–107 (2001).
Mayfield, L.D. & Corey, D.R. Automated synthesis of peptide nucleic acids (PNAs) and peptide nucleic acid–peptide conjugates. Anal. Biochem. 268, 401–404 (1999).
Elbashir, S.M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).
Crooke, S.T. Evaluating the mechanism of action of antiproliferative antisense drugs. Antisense Nucleic. Acid Drug Dev. 10, 123–126 (2000).
Editorial. Wither RNAi? Nat. Cell Biol. 5, 489–490 (2003).
Kastner, P. et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor isoforms A and B. EMBO J. 9, 1603–1614 (1990).
Misrahi, M. et al. Structure of the human progesterone receptor gene. Biochim. Biophys. Acta 1216, 289–292 (1993).
Hashimoto, S. et al. 5′-End SAGE for the analysis of transcriptional start sites. Nat. Biotechnol. 22, 1146–1149.
Yamashita, R. et al. DBTSS: DataBase of Human Transcription Start Sites, progress report 2006. Nucleic Acids Res. D86–D89 (2006).
Acknowledgements
We thank S. Younger, D. Ly and B. Armitage for comments. This work was supported by the National Institutes of Health (grants NIGMS 60642 and 73042 to D.R.C.), the High-Q Foundation, Applied Biosystems and the Robert A. Welch Foundation (grant I-1244 to D.R.C.).
Author information
Authors and Affiliations
Contributions
D.R.C. wrote the manuscript and supervised the experiments. B.A.J. and J.H. developed the protocols and assisted in writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
This work was partially funded by Applied Biosystems.
Rights and permissions
About this article
Cite this article
Janowski, B., Hu, J. & Corey, D. Silencing gene expression by targeting chromosomal DNA with antigene peptide nucleic acids and duplex RNAs. Nat Protoc 1, 436–443 (2006). https://doi.org/10.1038/nprot.2006.64
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.64
This article is cited by
-
Walking through the wonder years of artificial DNA: peptide nucleic acid
Molecular Biology Reports (2020)
-
Delivery of cell-penetrating peptide-peptide nucleic acid conjugates by assembly on an oligonucleotide scaffold
Scientific Reports (2015)
-
A sequence-specific threading tetra-intercalator with an extremely slow dissociation rate constant
Nature Chemistry (2011)
-
Antisense transcripts are targets for activating small RNAs
Nature Structural & Molecular Biology (2008)
-
Progress and prospects: RNA-based therapies for treatment of HIV infection
Gene Therapy (2007)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.