Abstract
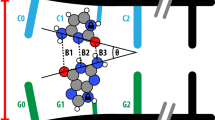
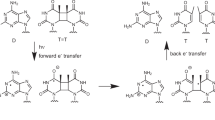
The various conformations of DNA are thought to have important biological roles. Investigation of the local DNA conformational changes associated with biological events is therefore essential to an understanding of the functions of DNA. We have reported the photoreactivities of 5-halouracil in the five characteristic local DNA structures: the A, B and Z forms, protein-induced DNA kinks and the G-quadruplex form. These studies demonstrate the detailed relationships between the local DNA structures and the photochemical products of photoinduced hydrogen abstraction by the resulting uracil-5-yl radicals, and show that this photochemical method can be used to detect DNA structures. Here, we describe in detail procedures that have been developed in our laboratory for probing DNA conformations by product analysis of photoirradiated 5-halouracil–containing DNA. The protocol includes the preparation of 5-halouracil–containing DNA and the characterization of the photoproducts, and it can be completed in 2 weeks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Travers, A.A. DNA conformation and protein binding. Annu. Rev. Biochem. 58, 427–452 (1989).
Wang, A.H. et al. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature 282, 680–686 (1979).
Sinden, R.R. DNA Structure and Function (Academic Press, New York, 1994).
Rich, A. & Zhang, S. Timeline: Z-DNA: the long road to biological function. Nat. Rev. Genet. 4, 566–572 (2003).
Hackett, J.A., Feldser, D.M. & Greider, C.W. Telomere dysfunction increases mutation rate and genomic instability. Cell 106, 275–286 (2001).
Lei, M., Podell, E.R., Baumann, P. & Cech, T.R. DNA self-recognition in the structure of Pot1 bound to telomeric single-stranded DNA. Nature 426, 198–203 (2003).
Neidle, S. & Parkinson, G.N. Telomere maintenance as a target for anticancer drug discovery. Nat. Rev. Drug Discov. 1, 383–393 (2002).
Hurley, L.H. DNA and its associated processes as targets for cancer therapy. Nat. Rev. Cancer 2, 188–200 (2002).
Mergny, J.-L. et. al. Natural and pharmacological regulation of telomerase. Nucleic Acids Res. 30, 839–865 (2002).
Hahn, W.C. et al. Inhibition of telomerase limits the growth of human cancer cells. Nat. Med. 5, 1164–1170 (1999).
Zahler, A.M., Williamson, J.R., Cech, T.R. & Prescott, D.M. Inhibition of telomerase by G-quartet DNA structures. Nature 350, 718–720 (1991).
Shay, J.W., Zou, Y., Hiyama, E. & Wright, W.E. Telomerase and cancer. Hum. Mol. Genet. 10, 677–685 (2001).
Liu, R. et al. Regulation of CSF1 promoter by the SWI/SNF-like BAF complex. Cell 106, 309–318 (2001).
Blackburn, E.H. Structure and function of telomeres. Nature 350, 569–573 (1991).
Kim, J.L., Nikolov, D.B. & Burley, S.K. Co-crystal structure of TBP recognizing the minor groove of a TATA element. Nature 365, 520–527 (1993).
Nikolov, D.B. et al. Crystal structure of a human TATA box-binding protein/TATA element complex. Proc. Natl. Acad. Sci. USA 93, 4862–4867 (1996).
Kim, Y., Geiger, J.H., Hahn, S. & Sigler, P.B. Crystal structure of a yeast TBP/TATA-box complex. Nature 365, 512–520 (1993).
Werner, M.H. et al. The solution structure of the human ETS1-DNA complex reveals a novel mode of binding and true side chain intercalation. Cell 83, 761–771 (1995).
Schumacher, M.A., Choi, K.Y., Zalkin, H. & Brennan, R.G. Crystal structure of LacI member, PurR, bound to DNA: minor groove binding by alpha helices. Science 266, 763–770 (1994).
Werner, M.H., Huth, J.R., Gronenborn, A.M. & Clore, G.M. Molecular basis of human 46X,Y sex reversal revealed from the three-dimensional solution structure of the human SRY-DNA complex. Cell 81, 705–714 (1995).
Zimmerman, S.B. & Pheiffer, B.H. A direct demonstration that the ethanol-induced transition of DNA is between the A and B forms: an X-ray diffraction study. J. Mol. Biol. 135, 1023–1027 (1979).
Reid, D.G. Proton nuclear Overhauser effect study of the structure of a deoxyoligonucleotide duplex in aqueous solution. Biochemistry 22, 2019–2025 (1983).
Clore, G.M. & Gronenborn, A.M. Probing the three-dimensional structures of DNA and RNA oligonucleotides in solution by nuclear Overhauser enhancement measurements. FEBS Lett. 179, 187–198 (1985).
Xu, Y. & Sugiyama, H. Photochemical approach to probing different DNA structure. Angew. Chem. Int. Ed. Engl. 45, 1354–1362 (2006).
Sugiyama, H., Tsutsumi, Y. & Saito, I. Highly sequence-selective photoreaction of 5-bromouracil-containing deoxyhexanucleotides. J. Am. Chem. Soc. 112, 6720–6721 (1990).
Sugiyama, H., Tsutsumi, Y., Fujimoto, K. & Saito, I. Photoinduced deoxyribose C2′ oxidation in DNA. Alkali-dependent cleavage of erythrose-containing sites via a retroaldol reaction. J. Am. Chem. Soc. 115, 4443–4447 (1993).
Sugiyama, H. et al. Evidence for intrastrand C2′ hydrogen abstraction in photoirradiation of 5-halouracil-containing oligonucleotides by using stereospecifically C2′-deuterated deoxyadenosine. Tetrahedr. Lett. 37, 1805–1808 (1996).
Sugiyama, H., Fujimoto, K. & Saito, I. Preferential C1′ hydrogen abstraction by an uracilyl radical in a DNA-RNA hybrid. Tetrahedr. Lett. 38, 8057–8060 (1997).
Kawai, K., Saito, I. & Sugiyama, H. Conformation-dependent photochemistry of 5-halouracil-containing DNA: stereospecific 2′-hydroxylation of deoxyribose in Z-form DNA. J. Am. Chem. Soc. 121, 1391–1392 (1999).
Oyoshi, T., Kawai, K. & Sugiyama, H. Efficient C2′-hydroxylation of deoxyribose in protein-induced Z-form DNA. J. Am. Chem. Soc. 125, 1526–1531 (2003).
Xu, Y., Ikeda, R. & Sugiyama, H. 8-Methylguanosine: a powerful Z-DNA stabilizer. J. Am. Chem. Soc. 125, 13519–13524 (2003).
Oyoshi, T., Wang, A.H.-J. & Sugiyama, H. Photoreactivity of 5-iodouracil-containing DNA-Sso7d complex in solution: the protein-induced DNA kink causes intrastrand hydrogen abstraction from the 5-methyl of thymine at the 5′ side. J. Am. Chem. Soc. 124, 2086–2087 (2002).
Xu, Y. & Sugiyama, H. Highly efficient photochemical 2′-deoxyribonolactone formation at the diagonal loop of a 5-iodouracil-containing antiparallel G-quartet. J. Am. Chem. Soc. 126, 6274–6279 (2004).
Weitzmann, M.N., Woodford, K.J. & Usdin, K. The development and use of a DNA polymerase arrest assay for the evaluation of parameters affecting intrastrand tetraplex formation. J. Biol. Chem. 271, 20958–20964 (1996).
Nevins, J.R. The Rb/E2F pathway and cancer. Hum. Mol. Genet. 10, 699–703 (2001).
Willis, M.C. et al. Photocrosslinking of 5-iodouracil-substituted RNA and DNA to proteins. Science 262, 1255–1257 (1993).
Ito, T. & Rokita, S.E. Excess electron transfer from an internally conjugated aromatic amine to 5-bromo-2′-deoxyuridine in DNA. J. Am. Chem. Soc. 125, 11480–11481 (2003).
Manetto, A., Breeger, S., Chatgilialoglu, C. & Carell, T. Complex sequence dependence by excess-electron transfer through DNA with different strength electron acceptors. Angew. Chem. Int. Ed. Engl. 45, 318–321 (2005).
Watanabe, T., Bando, T., Xu, Y., Tashiro, R. & Sugiyama, H. Efficient generation of 2′-deoxyuridin-5-yl at 5′-(G/C)AA(X)U(X)U-3′ (X = Br, I) sequences in duplex DNA under UV irradiation. J. Am. Chem. Soc. 127, 44–45 (2005).
Tashiro, R., Wang, A.H.-J. & Sugiyama, H. Photoreactivation of DNA by an archaeal nucleoprotein Sso7d. Proc. Natl. Acad. Sci. USA 103, 16655–16659 (2006).
Sugiyama, H. New synthetic method of 5-formyluracil-containing oligonucleotides and their melting behavior. Tetrahedr. Lett. 37, 9067–9070 (1996).
Sugiyama, H. et al. Synthesis, structure and thermodynamic properties of 8-methylguanine-containing oligonucleotides: Z-DNA under physiological salt conditions. Nucleic Acids Res. 24, 1272–1278 (1996).
Berger, I. et al. Spectroscopic characterization of a DNA-binding domain, Z, from the editing enzyme, dsRNA adenosine deaminase: evidence for left-handed Z-DNA in the Z-DNA complex. Biochemistry 37, 13313–13315 (1998).
Gao, Y.-G. et al. The crystal structure of the hyperthermophile chromosomal protein Sso7d bound to DNA. Nat. Struct. Biol. 5, 782–785 (1998).
Schwartz, T., Rould, M.A., Lowenhaupt, K., Herbert, A. & Rich, A. Crystal structure of the Zα domain of the human editing enzyme ADAR1 bound to left-handed Z-DNA. Science 284, 1841–1845 (1999).
Wang, Y. & Patel, D.J. Solution structure of the human telomeric repeat d[AG3(T2AG3)3] G-tetraplex. Structure 1, 263–282 (1993).
Parkinson, G.N., Lee, M.P. & Neidle, S. Crystal structure of parallel quadruplexes from human telomeric DNA. Nature 417, 876–880 (2002).
Acknowledgements
This study was partly supported by a Grant-in-Aid for Priority Research from the Ministry of Education, Science, Sports, and Culture and a grant from Solution Oriented Research for Science and Technology of Japan Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Xu, Y., Tashiro, R. & Sugiyama, H. Photochemical determination of different DNA structures. Nat Protoc 2, 78–87 (2007). https://doi.org/10.1038/nprot.2006.467
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.467
This article is cited by
-
Probing of G-Quadruplex Structures via Ligand-Sensitized Photochemical Reactions in BrU-Substituted DNA
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.