Abstract
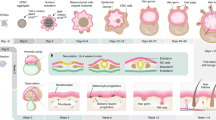
In this report we describe the development of a standardized three-dimensional (3D) system of the human oral mucosa based on an immortalized human oral keratinocyte cell line (OKF6/TERT-2). The procedure takes approximately 2–3 weeks to complete and includes three main stages: preparation of collagen-embedded fibroblasts, addition of the mucosal component and airlifting of cultures to ensure adequate differentiation/stratification. This procedure results in a multilayer epithelial structure in which layers are organized similarly to the cells in native oral mucosa. Specifically, this model system consists of a stratum basale, having one layer of columnar to round cells, a relatively flattened stratum spinosum and stratum granulosum, and a non-keratinizing stratum corneum. This 3D system resembles the commercially available system based on the cell line TR146 (SkinEthic), with the exception that our model system does not contain dyskeratotic changes and has a submucosal component, and thus better represents the normal human mucosa and submucosa.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Steele, C. & Fidel, P.L., Jr. Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans. Infect. Immun. 70, 577–583 (2002).
Dongari-Bagtzoglou, A.I. & Kashleva, H. Candida albicans triggers interleukin-8 secretion by oral epithelial cells. Microb. Pathogen. 34, 169–177 (2003).
Dongari-Bagtzoglou, A.I. & Kashleva, H. Granulocyte–macrophage colony-stimulating factor responses of oral epithelial cells to Candida albicans. Oral Microbiol. Immunol. 18, 165–170 (2003).
Dongari-Bagtzoglou, A.I., Kashleva, H. & Villar, C.C. Bioactive interleukin-1α is cytolytically released from Candida albicans-infected oral epithelial cells. Med. Mycol. 42, 531–541 (2004).
Krisanaprakornkit, S., Weinberg, A., Perez, C.N. & Dale, B.A. Expression of the peptide antibiotic human beta-defensin 1 in cultured gingival epithelial cells and gingival tissue. Infect. Immun. 66, 4222–4228 (1998).
Oda, D. & Watson, E. Human oral epithelial cell culture I. Improved conditions for reproducible culture in serum-free medium. In Vitro Cell. Dev. Biol. 26, 589–95 (1990).
Dongari-Bagtzoglou, A., Wen, K. & Lamster, I.B. Candida albicans triggers interleukin-6 and interleukin-8 responses by oral fibroblasts in vitro. Oral Microbiol. Immunol. 14, 364–370 (1999).
Dongari-Bagtzoglou, A.I. & Kashleva, H. Development of a novel three-dimensional in vitro model of oral Candida infection. Microb. Pathogen. 40, 271–278 (2006).
Cox, G., Gauldie, J. & Jordana, M. Bronchial epithelial cell-derived cytokines (G-CSF and GM-CSF) promote the survival of peripheral blood neutrophils in vitro. Am. J. Respir. Cell. Mol. Biol. 7, 507–513 (1992).
Devalia, J.L., Bayram, H., Abdelaziz, M.M., Sapsford, R.J. & Davies, R.J. Differences between cytokine release from bronchial epithelial cells of asthmatic patients and non-asthmatic subjects: effect of exposure to diesel exhaust particles. Int. Arch. Allergy Immunol. 118, 437–439 (1999).
Feucht, E.C., DeSanti, C.L. & Weinberg, A. Selective induction of human beta-defensin mRNAs by Actinobacillus actinomycetemcomitants in primary and immortalized oral epithelial cells. Oral Microbiol. Immunol. 18, 359–363 (2003).
Radyuk, S.N., Mericko, P.A., Popova, T.G., Grene, E. & Alibek, K. In vitro-generated respiratory mucosa: a new tool to study inhalational anthrax. Biochem. Biophys. Res. Commun. 305, 624–632 (2003).
Sacks, P.G. Cell, tissue and organ culture as in vitro models to study the biology of squamous cell carcinomas of the head and neck. Cancer Metast. Rev. 15, 27–51 (1996).
Kimball, J.R., Nittayananta, W., Klausner, M., Chung, W.O. & Dale, B.A. Antimicrobial barrier of an in vitro oral epithelial model. Arch. Oral Biol. 51, 775–783 (2006).
Schaller, M., Mailhammer, R., Grassl, G., Sander, C.A., Hube, B. & Korting, H.C. Infection of human oral epithelia with Candida species induces cytokine expression correlated to the degree of virulence. J. Invest. Dermatol. 118, 652–657 (2002).
Korting, H.C., Patzak, U., Schaller, M. & Maibach, H.I. A model of human cutaneous candidosis based on reconstructed human epidermis for the light and electron microscopic study of pathogenesis and treatment. J. Infect. 36, 259–267 (1998).
Maruguchi, T., Maruguchi, Y., Suzuki, S., Matsuda, K., Toda, K. & Isshiki, N. A new skin equivalent: keratinocytes proliferated and differentiated on collagen sponge containing fibroblasts. Plast. Reconstr. Surg. 93, 537–544 (1994).
Sugihara, H., Toda, S., Yonemitsu, N. & Watanabe, K. Effects of fat cells on keratinocytes and fibroblasts in a reconstructed rat skin model using collagen gel matrix culture. Br. J. Dermatol. 144, 244–253 (2001).
Kautsky, M.B., Fleckman, P. & Dale, B.A. Retinoic acid regulates oral epithelial differentiation by two mechanisms. J. Invest. Dermatol. 104, 546–553 (1995).
Mostefaoui, Y., Claveau, I. & Rouabhia, M. In vitro analyses of tissue structure and interleukin-1β expression and production by human oral mucosa in response to Candida albicans infections. Cytokine 25, 162–171 (2004).
Mostefaoui, Y., Bart, C., Frenette, M. & Rouabhia, M. Candida albicans and Streptococcus salivarius modulate IL-6, IL-8 and TNFα expression and secretion by engineered human oral mucosa cells. Cell. Microbiol. 6, 1085–1096 (2004).
Southgate, J., Williams, H.K., Trejdosiewicz, L.K. & Hodges, G.M. Primary culture of human oral epithelial cells. Growth requirements and expression of differentiated characteristics. Lab. Invest. 56, 211–223 (1987).
Dickson, M.A., Hahn, W.C., Ino, Y., Ronfard, V., Wu, J.Y., Weinberg, R.A., Louis, D.N., Li, F.P. & Rheinwald, J.G. Human keratinocytes that express hTERT and also bypass a p16 INK4a-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 20, 1436–1447 (2000).
Parenteau, N. Skin equivalents. in Keratinocyte Methods (eds. Leigh, I. & Watt, F.) 45–54 (Cambridge University Press, U.K., 1994).
Schon, M. & Rheinwald, J.G. A limited role for retinoic acid and retinoic acid receptors RARa and RARb in regulating Keratin 19 expression and keratinization in oral and epidermal keratinocytes. J. Invest. Dermatol. 107, 428–438 (1996).
Meyers, C., Frattini, M.G. & Laimins, L.A. Tissue culture techniques for the study of human papillomaviruses in stratified epithelia. in Cell Biology: A Laboratory Handbook (Ed. Celis, J.E.) 491–499 (Academic Press Inc., 1994).
Rouabhia, M. In vitro production and transplantation of immunologically active skin equivalents. Lab. Invest. 75, 503–517 (1996).
Dumont, S., Valladeu, J., Bechetoille, N., Gofflo, S. & Marechal, S. et al. When integrated in a subepithelial mucosal layer equivalent, dentritic cells keep their immature stage and their ability to replicate Type R5 HIV Type 1 strains in the absence of T cell subsets. AIDS Res. Hum. Retroviruses 20, 383–397 (2004).
Schaller, M., Korting, H.C., Schafer, W., Bastert, J., Chen, W. & Hube, B. Secreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosis. Mol. Microbiol. 34, 169–180 (1999).
Castillon, N. et al. Polarized expression of cystic fibrosis transmembrane conductance regulator and associated epithelial proteins during the regeneration of human airway surface epithelium in three-dimensional culture. Lab. Invest. 82, 989–998 (2002).
Costea, D.E., Johannessen, A.C. & Vintermyr, O.K. Fibroblast control on epithelial differentiation is gradually lost during in vitro tumor progression. Differentiation 73, 134–141 (2005).
Downer, C.S. & Speight, P.M. E-cadherin expression in normal, hyperplastic and malignant oral epithelium. Eur. J. Cancer B. Oral Oncol. 29, 303–305 (1993).
Gasparoni, A., Fonzi, L., Schneider, G.B., Wertz, P.W., Johnson, G.K. & Squier, C.A. Comparison of differentiation markers between normal and two squamous cell carcinoma cell lines in culture. Arch. Oral Biol. 49, 653–664 (2004).
Waelti, E.R. et al. Co-culture of human keratinocytes on post-mitotic human dermal fibroblast feeder cells: production of large amounts of interleukin 6. J. Invest. Dermatol. 98, 805–808 (1992).
Squier, C.A. & Finkelstein, M.W. Oral mucosa. in Ten Cate's Oral Histology, 6th Edition (ed. Nanci, A.) (Mosby Inc., Orlando, FL, 2003).
Barreca, A. et al. In vitro paracrine regulation of human keratinocyte growth by fibroblast-derived insulin-like growth factors. J. Cell. Physiol. 151, 262–268 (1992).
Villar, C.C., Kashleva, H., Mitchell, A.P. & Dongari-Bagtzoglou, A.I. Invasive phenotype of Candida albicans affects the host proinflammatory response to infection. Infect. Immun. 273, 4588–4595 (2005).
de Brugerolle, A. et al. Predictivity of an in vitro model for acute and chronic skin irritation (SkinEthic) applied to the testing of topical vehicles. Cell Biol. Toxicol. 15, 121–135 (1999).
Reichart, P.A., Philipsen, H.P., Schmidt-Westhausen, A. & Samaranayake, L.P. Pseudomembranous oral candidiasis in HIV infection: ultrastructural findings. J Oral Pathol. Med. 24, 276–281 (1995).
Acknowledgements
This study was supported by USPHS Research Grant RO1 DE13986 to ADB from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Dongari-Bagtzoglou, A., Kashleva, H. Development of a highly reproducible three-dimensional organotypic model of the oral mucosa. Nat Protoc 1, 2012–2018 (2006). https://doi.org/10.1038/nprot.2006.323
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.323
This article is cited by
-
In vitro three-dimensional organotypic culture models of the oral mucosa
In Vitro Cellular & Developmental Biology - Animal (2021)
-
Characterization of an ester-based core-multishell (CMS) nanocarrier for the topical application at the oral mucosa
Clinical Oral Investigations (2021)
-
Role of glucosyltransferase R in biofilm interactions between Streptococcus oralis and Candida albicans
The ISME Journal (2020)
-
Mechanism and significance of apoptosis of the immortalized human oral mucosal epithelial cells established by Lentivirus-mediated hTERT
Molecular Biology Reports (2020)
-
Anisomycin, a JNK and p38 activator, suppresses cell–cell junction formation in 2D cultures of K38 mouse keratinocyte cells and reduces claudin-7 expression, with an increase of paracellular permeability in 3D cultures
Histochemistry and Cell Biology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.