Abstract
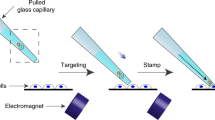
Diolistic labeling is a highly efficient method for introducing dyes into cells using biolistic techniques. The use of lipophilic carbocyanine dyes, combined with particle-mediated biolistic delivery using a hand-held gene gun, allows non-toxic labeling of multiple cells in both living and fixed tissue. The technique is rapid (labeled cells can be visualized in minutes) and technically undemanding. Here, we provide a detailed protocol for diolistic labeling of cultured human embryonic kidney 293 cells and whole brain using a hand-held gene gun. There are four major steps: (i) coating gold microcarriers with one or more dyes; (ii) transferring the microcarriers into a cartridge to make a bullet; (iii) preparation of cells or intact tissue; and (iv) firing the microcarriers into cells or tissue. The method can be readily adapted to other cell types and tissues. This protocol can be completed in less than 1 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Magrassi, L., Purves, D. & Lichtman, J.W. Fluorescent probes that stain living nerve terminals. J. Neurosci. 7, 1207–1214 (1987).
Stephens, D.J. & Pepperkok, R. The many ways to cross the plasma membrane. Proc. Natl. Acad. Sci. USA 98, 4295–4298 (2001).
Chalfie, M., Tu, Y., Euskirchen, G., Ward, W.W. & Prasher, D.C. Green fluorescent protein as a marker for gene expression. Science 263, 802–805 (1994).
van den Pol, A.N. & Ghosh, P.K. Selective neuronal expression of green fluorescent protein with cytomegalovirus promoter reveals entire neuronal arbor in transgenic mice. J. Neurosci. 18, 10640–10651 (1998).
Lo, D.C., McAllister, A.K. & Katz, L.C. Neuronal transfection in brain slices using particle-mediated gene transfer. Neuron 13, 1263–1268 (1994).
Honig, M.G. & Hume, R.I. Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term cultures. J. Cell Biol. 103, 171–187 (1986).
Honig, M.G. & Hume, R.I. Formation of synapses by sympathetic preganglionic neurons. Soc. Neurosci. Abstr. 13, 425 (1987).
Gan, W.B., Grutzendler, J., Wong, W.T., Wong, R.O.L. & Lichtman, J.W. Multicolor “diolistic” labeling of the nervous system using lipophilic dye combinations. Neuron 27, 219–225 (2000).
Gan, W.B. & Lichtman, J.W. Synaptic segregation at the developing neuromuscualr junction. Science 282, 1508–1511 (1998).
Godement, P., Vanselow, J., Thanos, S. & Bonhoeffer, F.A. Study in developing visual systems with a new method of staining neurons and their processes in fixed tissue. Development 4, 697–713 (1987).
Liu, D.W. & Westerfield, M. The formation of terminal fields in the absence of competitive interactions among primary motorneurons in the zebrafish. J. Neurosci. 12, 3947–3959 (1990).
Burkhalter, A. & Bernardo, K.L. Organization of corticocortical connections in human visual cortex. Proc. Natl. Acad. Sci. USA 86, 1071–1075 (1989).
Cline, H.T., Edwards, J.E., Rajan, I., Wu, G.Y. & Zou, D.J. in Imaging Neurons, A Laboratory Manual (ed. R. Yuste, F. Lanni and A. Konnerth) (Cold Spring Harbor Press, Cold Spring Harbor, New York, 2000).
Pearson, R.A., Luneborg, N.L., Becker, D.L. & Mobbs, P. Gap junctions modulate interkinetic nuclear movement in retinal progenitor cells. J. Neurosci. 25, 10803–10814 (2005).
O'Brien, J. & Unwin, N. Organization of spines on the dendrites of Purkinje cells. Proc. Natl. Acad. Sci. USA 103, 1575–1580 (2006).
O'Brien, J.A., Holt, M., Whiteside, G., Lummis, S.C. & Hastings, M.H. Modifications to the hand-held gene gun: improvements for in vitro biolistic transfection of organotypic neuronal tissue. J. Neurosci. Methods 112, 57–64 (2001).
O'Brien, J.A. & Lummis, S.C.R. Biolistic transfection of neuronal cultures using a hand-held gene gun. Nat. Protocols 1, 977–981 (2006).
Sanford, J.C., Smith, F.C. & Russell, J.A. Optimizing the biolistic process for different biological applications. Methods 217, 483–509 (1993).
Acknowledgements
This work was supported by the Medical Research Council and the Wellcome Trust. S.C.R.L. holds a Wellcome Trust Senior Research Fellowship in Basic Biomedical Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
O'Brien, J., Lummis, S. Diolistic labeling of neuronal cultures and intact tissue using a hand-held gene gun. Nat Protoc 1, 1517–1521 (2006). https://doi.org/10.1038/nprot.2006.258
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.258
This article is cited by
-
Femtosecond optical transfection of individual mammalian cells
Nature Protocols (2013)
-
Age-dependent regulation of synaptic connections by dopamine D2 receptors
Nature Neuroscience (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.