Abstract
Dopamine D3 receptor antagonists exert pro-cognitive effects in both rodents and primates. Accordingly, this study compared the roles of dopamine D3 vs D2 receptors in social novelty discrimination (SND), which relies on olfactory cues, and novel object recognition (NOR), a visual-recognition task. The dopamine D3 receptor antagonist, S33084 (0.04–0.63 mg/kg), caused a dose-related reversal of delay-dependent impairment in both SND and NOR procedures in adult rats. Furthermore, mice genetically deficient in dopamine D3 receptors displayed enhanced discrimination in the SND task compared with wild-type controls. In contrast, acute treatment with the preferential dopamine D2 receptor antagonist, L741,626 (0.16–5.0 mg/kg), or with the dopamine D3 agonist, PD128,907 (0.63–40 μg/kg), caused a dose-related impairment in performance in rats in both tasks after a short inter-trial delay. Bilateral microinjection of S33084 (2.5 μg/side) into the prefrontal cortex (PFC) of rats increased SND and caused a dose-related (0.63–2.5 μg/side) improvement in NOR, while intra-striatal injection (2.5 μg/side) had no effect on either. In contrast, bilateral microinjection of L741,626 into the PFC (but not striatum) caused a dose-related (0.63–2.5 μg/side) impairment of NOR. These observations suggest that blockade of dopamine D3 receptors enhances both SND and NOR, whereas D3 receptor activation or antagonism of dopamine D2 receptor impairs cognition in these paradigms. Furthermore, these actions are mediated, at least partly, by the PFC. These data have important implications for exploitation of dopaminergic mechanisms in the treatment of schizophrenia and other CNS disorders, and support the potential therapeutic utility of dopamine D3 receptor antagonism.
Similar content being viewed by others
INTRODUCTION
The dopaminergic system has a key role in a diverse array of physiological processes including neuroendocrine function, motor behavior, emotion, and cognitive function. Similarly, dysfunction of dopaminergic pathways and the closely related dopamine D2 and D3 receptors are implicated in the pathophysiology of Parkinson's disease, ADHD, and schizophrenia, as well as substance abuse (Heidbreder and Newman, 2010; Joyce, 2001). A high density of dopamine D2 receptors occurs in rodent and primate caudate putamen and nucleus accumbens, but they are also found in many other brain regions at lower levels (Heidbreder and Newman, 2010; Joyce, 2001; Sokoloff et al, 2006). Dopamine D3 receptors are less abundant than their D2 counterparts in the brain and are principally located in mesolimbic regions like the ventral striatum, islands of Calleja, nucleus accumbens, globus pallidus, and thalamus, but they are also found in the frontal and other cortical regions as well as the cerebellum (Herroelen et al, 1994; Meador-Woodruff et al, 1996; Sokoloff et al, 2006; Sokoloff et al, 1990). PET studies in mice and baboons, using the preferential D3 ligand, [11C](+)-PHNO, in the presence and absence of the D3 receptor antagonist, SB277,011, confirm the D3 receptor is more highly expressed than the D2 in the ventral pallidum, substantia nigra, thalamus, and habenula, while binding in the dorsal striatum is almost exclusively that of the D2 receptor (Rabiner et al, 2009).
Selective antagonists exist for characterizing the roles of dopamine D3 and D2 receptor subtypes (Boeckler and Gmeiner, 2006; Ehrlich et al, 2009; Joyce and Millan, 2005). The dopamine D3 receptor antagonists, SB277,011 and S33084, have high selectivity for the dopamine D3 over the D2 receptor (over 100-fold in ligand binding studies, Millan et al, 2000a, 2000b). Furthermore, L741,626 is currently the most preferential D2 receptor antagonist available, having between 15- and 40-fold selectivity for the rat dopamine D2 relative to the D3 receptor in radioligand binding (Cussac et al, 2000; Kulagowski et al, 1996; Millan et al, 2004; Reavill et al, 2000) and functional assays (Grundt et al, 2007), with both native and cloned receptors. No data are currently available in the mouse to confirm a similar selectivity. In the current behavioral studies, doses were carefully selected to enable predominant D2 receptor blockade to be achieved based on previous in-vivo work (Millan et al, 2004).
Together with dopamine D3 receptor agonists, such as PD128,907 (Pugsley et al, 1995), these are useful pharmacological tools to specifically characterize the location and function of these receptors (Boeckler and Gmeiner, 2006).
We previously demonstrated that the antagonist, S33138, which has ∼25-fold higher affinity for the D3 than the D2 receptor (Millan et al, 2008), has pro-cognitive effects in two rat models of cognition, social novelty discrimination (SND) and novel object recognition (NOR) (Millan et al, 2010). Interestingly, by using an extended dose range of S33138 the NOR data showed an inverted-U shape consistent with increasing occupancy of D2 receptor opposing the effect of D3 receptor antagonism on cognitive performance. Additionally, both S33138 and S33084 reversed the impairment in NOR produced by social isolation rearing of rat pups (a neurodevelopmental model of schizophrenia) while L741,626 was ineffective (Watson et al, 2011). These pro-cognitive effects of D3 receptor blockade are consistent with other reports that SB277,011 prevents scopolamine-induced impairment of spatial learning and memory in the Morris water maze (Laszy et al, 2005). Furthermore, in a rat social recognition paradigm, both S33084 and SB277,011 enhanced performance accompanied by elevation of frontal cortex acetylcholine overflow measured by microdialysis. In contrast, L741,626 had amnesic properties and no effect on cortical acetylcholine release (Millan et al, 2007). Furthermore microinjection of S33084 or SB277,011 into the prefrontal cortex (PFC), but not the nucleus accumbens or striatum, improved social recognition while L741,626 was without effect (Loiseau and Millan, 2009). Finally, mice genetically deficient in the D3 receptor show increased cognitive flexibility in the attentional set-shifting task and improved retention in a passive-avoidance test (Glickstein et al, 2005; Micale et al, 2010).
The current study further characterizes the opposing roles of dopamine D3 and D2 receptors using the selective D3 receptor antagonist, S33084, the preferential D2 receptor antagonist, L741,626, and the selective D3 agonist, PD128,907 in two rodent models of cognition (SND and NOR). This is the first systematic evaluation of the comparative role of dopamine D2 and D3 receptors in these behavioral tasks performed by altering the delay between trials such that rats are able (with short) or unable (with long delay) to discriminate; allowing characterization of drugs exerting both positive and detrimental effects on cognitive performance. Furthermore, we previously showed that social recognition is modulated, by dopamine D3 receptors in the PFC (Loiseau and Millan, 2009) probably reflecting its important role in the top-down control of cognitive processing, which is disrupted in schizophrenia (Baluch and Itti, 2011; Klinkenberg et al, 2011; Noudoost et al, 2010; Rossi et al, 2009; Sarter et al, 2009) and its well-establish role on working memory. Finally, amphetamine-induced improvement in NOR in an Fmr1 knockout mouse model of Fragile X syndrome correlated with the increase in PFC but not striatal dopamine release measured by microdialysis (Ventura et al, 2004). Hence, the role of the PFC in SND and NOR was investigated by discrete injection of selective compounds compared with the effect of control injections into the striatum, a region known to be involved in procedural memory but not object discrimination or social recognition. Finally, performance of mice deficient in dopamine D3 receptors (DRD3−/−) was examined in the SND task to further characterize the role of this receptor.
MATERIALS AND METHODS
Social Novelty Discrimination
The SND procedure was adapted from Terranova et al (2005) as previously used in this laboratory (Millan et al, 2010). Group-housed adult (240–260 g) and juvenile (21-day-old) male Wistar rats purchased from Janvier (France) adult rats were isolated 2 days before testing while juveniles were group-housed throughout. Half of the juveniles were marked on tail, coat, and head with non-odorous black dye. On the test day, a juvenile (unmarked ‘white’ or marked ‘black’, chosen in a pseudorandom manner) was placed into the adult home cage for 5 min (P1) and time spent by the adult in social investigation of the juvenile recorded. There was either no delay or a 30-min delay between the end of P1 and the juvenile being reintroduced for a second 5-min period (P2) together with another novel juvenile. During P2, the time spent investigating each juvenile (P2 novel and familiar) was recorded in seconds. The SND ratio (investigation of the novel juvenile/investigation of the familiar juvenile) was calculated for P2 measurements. Investigation times during P2 were analyzed by two-way ANOVA with exploration of novel and familiar juveniles as the repeated within-subjects factor and treatment as the between-subjects factor. SND ratio was analyzed using Mann–Whitney or Kruskal–Wallis followed by Dunn’s post hoc tests. Total investigation times during P1 and P2 were also analyzed by one-way ANOVA followed by Dunnett’s t or Fisher’s LSD post hoc tests. As several groups have shown the most pronounced interaction between adult and juvenile rats occurs within the first few minutes of P2 in the SND paradigm (Engelmann et al, 1995), supplementary information (Supplementary Figure S1) of the time course of the P2 interaction in an additional group of drug-free rats recorded 30 min after P1 shows that the SND ratio is suppressed in the first 2–3 min to the same extent as over 5 min. Therefore, the use of a 5-min P2 observation period does not mask an initial juvenile preference, validating the use of this protocol for all studies presented herein.
Mutant Mice
Mice genetically deprived of dopamine D3 receptors (D3 knockout mice; D3-KO) were obtained from the Jackson Laboratory (B6.129S4-Drd3Tm1Dac/J) (Accili et al, 1996). Homozygous mice were generated on a pure genetic background and were compared with C57BL/6J wild-type (WT) mice. Although several distinct lines of D2 receptor knockout mice are available they frequently develop hypertension and pituitary tumors (the latter resulting in elevated circulating glucocorticoid levels) and often show impaired motor function and gait (Holmes et al, 2004), features dependent on the background strain (Waddington et al, 2005). Although D2 knockout mice show impaired autoreceptor function, neither the synaptic level nor tissue dopamine content appear to alter, suggesting that developmental compensations including elevation in striatal dopamine transporter levels may compromise interpretation of which phenotypic alterations are specifically due to change in D2 receptor expression in these mice (Holmes et al, 2004; Waddington et al, 2005). Therefore, the current study used dopamine D2 receptor selective ligands instead of D2−/− mice. Several groups have used D2−/− mice for cognitive studies, and these show impaired reversal learning in an attentional set-shifting task (DeSteno and Schmauss, 2009), opposite to D3−/− which have improved reversal learning in a two choice perceptual discrimination task (Glickstein et al, 2005). Thus, future studies using D2 mutant mice (either knockout or overexpression; Li et al, 2011) would be valuable to confirm the current pharmacological evaluation.
Transgenic mice were isolated the day before the SND test, which was performed as described above.
Surgery
The surgical procedure for implanting bilateral stainless steel guide cannulae above the PFC or striatum was adapted from Chudasama and Robbins (2004) and was exactly as employed in our previous studies (Loiseau and Millan, 2009). As a control, equivalent microinjection was performed into the striatum because of the known very high level of expression of the dopamine D3 receptor in the striatum (Bouthenet et al, 1991; Diaz et al, 2000) and evidence that this area is not involved in the regulation of either SND or NOR.
Adult rats were anesthetized by an intraperitoneal (i.p.) injection of chloral hydrate (400 mg/kg, as 10 ml/kg) and placed in a stereotaxic frame (David Kopf Instruments, Phymep, Paris). Guide cannulae, consisting of two 22-gauge metal tubes (inner diameter: 0.39 mm) 1.5 mm apart (center to center), projecting 3.5 mm from a plastic pedestal for the PFC and 5.0 mm apart and projecting 5.0 mm for the striatum (Plastics One) were mounted on a stereotaxic frame and lowered at the following coordinates from Bregma: PFC; AP: +3.0, L: ±0.7, DV: −2.3 and striatum; AP: +0.5, L: ±2.5, DV: −4.0 (Paxinos and Watson, 1997). The cannulae were fixed with dental cement to adjacent stainless steel screw anchors. Stylets (Plastics One) placed in the guide cannulae prevented occlusion before drug injection. Animals were housed individually and allowed to recover for at least 1 week before testing.
Microinfusion
After post-operative recovery, rats were handled to minimize any stress associated with the drug infusion procedure. On the test day, rats were gently restrained while stylets were removed and replaced with a sterile 28-gauge (inner diameter: 0.18 mm, outer diameter: 0.36 mm) stainless steel injector extending 1.0 mm beyond the tip of the guide cannulae (Plastics One). Injectors were connected (Plastics One) to 10 μl precision syringes (Hamilton, Phymep, Paris) mounted in an infusion pump (Harvard Apparatus, Holliston, MA) and 1 μl drug or vehicle infused bilaterally over 2 min, 5 min before the P1 or familiarization trial. The injectors were left in place for 2 min before being removed.
Histology
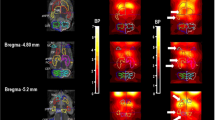
After behavioral testing, brains were removed and frozen for histological verification of the injection position from coronal brain sections according to the atlas of Paxinos and Watson (1997). Figure 1 illustrates the location of injections sites in the striatum and PFC.
Position of injection sites in the rat PFC (upper, +3.2 mm from Bregma) and striatum (lower diagram, +0.48 mm from Bregma) of rats used for behavioral studies on coronal sections taken from Paxinos and Watson (1997) together with a schematic representation of the injector.
Novel Object Recognition
Male Lister Hooded rats (Charles River, UK) housed in groups of four were used for NOR using a two trial object discrimination task adapted from Ennaceur and Delacour (1988) routinely in use in our laboratory (Bianchi et al, 2006; Bianchi et al, 2009; King et al, 2009; Lapiz et al, 2000). Rats (initially weighing ∼200 g) were habituated to the test arena (a 39.0 × 23.5 × 24.5 cm perspex cage) for 1 h, the day before each NOR test. On the test day, animals were re-acclimatized to the arena for 3 min, returned to their home cage while two identical objects (8 cm high, 5 cm diameter water-filled, plastic bottles covered with white masking tape) were secured by Blu-Tack to the floor in opposite corners of the arena (5 cm from the side and 10 cm from the back). The rat was allowed to explore both objects for 3 min during the first familiarization trial and the time(s) spent exploring each object recorded with stopwatches. Animals were returned to their home cage for a variable inter-trial interval before reintroduction into the cage with a copy of an original (familiar) object and one novel object (identical white masking taped plastic bottle covered with four prominent 1.2 cm black horizontal stripes) for a second 3-min trial. During the second choice trial, exploration of each object was recorded again separately. The location of the novel object was varied in a pseudorandom order within groups. Exploratory behavior was defined as sniffing, touching, and direct attention to the object, with active vibrissae while the nose was within 1 cm of the object. Climbing on or chewing the object was not considered exploratory behaviors and not recorded. Each study was performed in a separate cohort of rats (n=12/group) with each rat receiving every dose or combination of drugs in a pseudorandom order. This was done to minimize the influence of inter-individual variability and reduce the number of animals required. All behavioral studies were conducted by a single experienced examiner who was unaware of the treatment given. All behavioral apparatus was cleaned using 20% ethanol between trials and objects within trials to remove odor cues. Data were analyzed by two-way ANOVA with exploration of the novel and familiar objects the repeated within-subject factors and treatment the between-subject factor, with Bonferroni post hoc tests to determine significant differences in exploration between novel and familiar objects. As variation in exploration of individual objects between rats can confound interpretation, the choice trial raw data were converted to discrimination ratio (d2 score=(novel object−familiar object)/(novel object+familiar object)) values which were analyzed by two-way ANOVA followed by appropriate Dunnett's t or LSD post hoc tests to determine changes in object exploration pattern independent of actual object exploration times. All values are presented as mean±SEM and P<0.05 was considered significant. Surgical and microinfusion techniques were as described for the SND procedure except that anesthesia was induced with 3.5% isoflurane and maintained with 2% in 33% O2 and 66% N2O.
SND procedures conform to European (86/609-EEC) and French (87/848) decrees for the care and use of laboratory animals and NOR studies were conducted after approval by the University of Nottingham local ethical review committee and in accordance with the Home Office Animals (Scientific Procedures) 1986 ACT.
Drugs
S33084 (0.01–0.63 mg/kg) and L-741,626 (00.63–5 mg/kg) were dissolved in saline with lactic acid before adjusting to neutral pH. PD128,907 (0.63–40.0 μg/kg) was dissolved in saline. All drugs were injected (1 ml/kg, s.c.) 30 min before each test (P1 in SND and familiarization trial in NOR). In experiments where two drugs were administered, L741,626/vehicle was injected 45 min prior and S33084/vehicle 30 min before the first trial, S33084/vehicle 45 min prior followed by PD128,907/vehicle 30 min before the first trial. All drug doses are expressed in terms of the base. For the microinfusion studies S33084 was dissolved in artificial cerebrospinal fluid (aCSF) containing 10% hydroxypropyl-β-cyclodextrine (Sigma, Chesnes, France) and L741,626 in aCSF containing 5% dimethyl sulfoxide (Riedel-de Haën, Germany) and 5% cremophor EL (Sigma). All drugs were provided by Institut de Recherches Servier.
RESULTS
Effect of Dopamine D3 and D2 Antagonists on Delay-Dependent Impairment of SND
As expected by using a 30-min delay between P1 and P2 in the SND task, vehicle-treated rats spent an equal time investigating the novel and familiar juvenile during P2, consistent with an inability to perform social discrimination with this inter-trial delay (Figure 2a). Following S33084 injection, two-way repeated measures ANOVA on the P2 data showed a significant juvenile × treatment interaction (F(3,19)=5.15, P<0.01). The preferential dopamine D3 antagonist, S33084, caused a progressive increase in social discrimination (H=9.778, 3, N=23, P<0.05) which reached significance from vehicle (Figure 2b, P<0.05) with the highest dose (0.63 mg/kg). Moreover, since S33084 did not significantly alter the total investigation of the juveniles during either P1 or P2 (Table 1), this increase in SND was clearly due to a redistribution of investigative behavior to the novel rat rather than a non-specific change in exploratory behavior or sedation.
Dose–response effect of, and interaction between the preferential dopamine D3 receptor antagonist, S33084, and the preferential dopamine D2 receptor antagonist, L741,626 on social novelty discrimination (SND) in the rat. Data in (a, c, and e) show time spent investigating the novel compared with the familiar juvenile in P2 following a 30-min inter-trial interval (mean±SEM of juvenile exploration). ***P<0.001 from the novel juvenile at the same dose in the same treatment group (Bonferroni post hoc). Panels (b), (d) and (f) show the SND ratio (novel/familiar) for the same behaviour, *P<0.01 compared with vehicle, **P<0.01 compared with vehicle/vehicle group, ++P<0.01 compared with vehicle/S33084 group. Numbers in parentheses indicate number of rats in each group. Note the D3 receptor antagonist restores a time delay-induced reduction in SND (a, b) which is prevented by the D2 receptor antagonist (e, f), while the latter has no effect on its own at this inter-trial internal (c, d).
Treatment with the preferential dopamine D2 receptor antagonist, L741,626, did not significantly affect social discrimination after a 30-min delay (Figure 2d) in SND. At all doses, rats spent an equal time investigating the novel and familiar juvenile (Figure 2c), such that ANOVA showed there was no significant juvenile × treatment interaction. However, it should be noted that total investigative activity during both P1 and P2 was reduced in a dose-related manner by L741,626 (Table 1, P1: F(3,20)=6.4, P<0.01, P2: F(3,20)=15.15, P<0.001).
The reversal of the social discrimination impairment produced by S33084 (0.63 mg/kg) using a 30-min inter-trial delay was blocked by pretreatment with L741,626 (2.5 mg/kg) (Figure 2f, S33084 treatment: F(1,31)=6.37, P<0.05, L741,626 treatment: F(1,31)=5.82, P<0.05, S33084 × L741,626 interaction: F(1,31)=4.65, P<0.05). In this group, there was significant juvenile × S33084 interaction (F(1,31)=6.94, P<0.05) and a significant juvenile × L741,626 interaction (F(1,31)=6.94, P<0.05). Thus, groups treated with vehicle/vehicle and L741,626/vehicle spent an equal time investigating both juveniles while the vehicle/S33084-treated group spent significantly longer investigating the novel than the familiar juvenile (Figure 2e). Interestingly, when given in combination (L741,626/S33084) the preferential exploration of the novel juvenile was prevented. Neither drug at the doses used had any significant effect on total investigation activity during P1 (Table 1). However, during P2, while S33084 treatment had no effect, L741,626 did slightly reduce total investigatory activity which reached significance, as shown in Table 1 (S33084: not significant; L741,626: F(1,31)=6.51, P<0.05; S33084 × L741,626: not significant).
Effect of Dopamine D2 and D3 Antagonists and a Dopamine D3 Agonist on SND with No Inter-Trial Interval
With no delay between P1 and P2 in the SND task vehicle-treated rats naturally spend more time exploring the novel than the familiar juvenile, as seen in Figure 3a. The preferential dopamine D3 receptor antagonist, S33084, had no significant affect on this distribution of investigation between the juveniles (object × S33084 treatment; not significant), such that with all doses rats preferentially explored the novel over the familiar juvenile (Figure 3b). Furthermore, the total social investigation during P1 and P2 was unaffected by S33084 (Table 1).
Dose–response effects of the preferential dopamine D3 receptor antagonist, S33084, the preferential dopamine D2 receptor antagonist, L741,626 and the dopamine D3 agonist, PD128,907, on social novelty discrimination (SND) with no inter-trial delay in the rat. Data in (a, c, and e) show time spent investigating the novel compared with the familiar juvenile in P2 (mean±SEM of juvenile exploration). *P<0.05, **P<0.01, and ***P<0.001 from the novel juvenile at the same dose in the same treatment group (Bonferroni post hoc following ANOVA). The SND ratio (novel/familiar) is shown in (b), (d), and (f). **P<0.05 and ***P<0.001 compared with vehicle. Numbers in parentheses indicate the number of rats in each group. Note that while the D3 receptor antagonist has no effect on SND, both the D2 receptor antagonist and D3 receptor agonist impair recognition of the novel juvenile in P2.
The preferential dopamine D2 receptor antagonist, L741,626, caused a progressive impairment in the ability of rats to discriminate the novel juvenile in this task (Figure 3d, H=16.11, 3, N=29, P<0.01) which reached significance with the highest dose (2.5 mg/kg). Two-way ANOVA performed on the P2 data showed a significant juvenile × L741,626 interaction (F(3,25)=7.03, P<0.01), and post hoc analysis showed that after the highest dose (2.5 mg/kg) of L741,626 rats spent an equal time investigating each juvenile (Figure 3c). Although L741,626 had a significant effect on total social investigatory behavior during P1 (F(3,25)=4.30, P<0.05), this was not dose dependent and no significant effect was seen during P2 (Table 1).
After administration of the dopamine D3 receptor agonist, PD128,907, two-way repeated measures ANOVA performed on the investigation of the novel and familiar juvenile during P2 showed a significant juvenile × PD128,907 treatment interaction (F(4,29)=7.55, P<0.001). Post hoc analysis showed that there was no significant difference between the amount of investigation of the novel or familiar juvenile with the two highest doses tested (10 and 40 μg/kg, Figure 3e). The ratio data (Figure 3f) confirmed a significant treatment effect (H=14.95, 4, N=34, P<0.01); however, only the 10.0-μg/kg dose significantly reduced the discrimination ratio from that in vehicle-treated rats (P<0.05). PD128,907 had no significant effect on total social investigatory activity in either trial.
Effect of Bilateral Microinjection of Dopamine D2 and D3 Antagonists into the PFC or Striatum on SND
Bilateral microinjection of the preferential dopamine D3 receptor antagonist, S33084 (2.5 μg/side), into the PFC had no significant effect on total investigatory activity in either P1 or P2 (Table 1). After microinjection into the PFC S33084 caused a significant juvenile × treatment interaction (F(1,9)=30.24, P<0.001), such that it significantly improved SND after a 30-min delay (Figure 4a and b, U=0.00, P<0.01). In contrast, bilateral injection of S33084 (2.5 μg/side) into the striatum had no significant effect on SND such that rats showed equal investigation of both juveniles (Figure 4c) and the SND ratio remained at chance level for both vehicle and S33084 groups (Figure 4d). As with microinjections into the PFC, injection into the striatum had no significant effect on total time spent investigating juveniles during P1 or P2 (Table 1).
Effect of bilateral microinjection of the preferential dopamine D3 receptor antagonist S33084 into the prefrontal cortex (a) or striatum (c) on social novelty discrimination (SND) in the rat. Bars show juvenile exploration (sec, mean±SEM) following a 30-min inter-trial interval. ***P<0.001 from the novel juvenile in the same treatment group (Bonferroni post hoc following ANOVA). Panels (b) and (d) show the SND ratio (novel/familiar) following microinjection of S33084 (2.5 μg/side) into the prefrontal cortex and striatum, respectively. **P<0.01 compared with vehicle. Numbers in parentheses indicate the number of rats in each group. Note that S33084 only attenuated SND when injected into the prefrontal cortex and not into the striatum.
Performance of Mutant DRD3−/− Mice in the SND Task and the Effect of a Dopamine D3 Receptor Antagonist
After a 30-min delay, mice deficient in dopamine D3 receptors spent significantly more time investigating the novel than the familiar juvenile (Figure 5a, juvenile × genotype: F(1,10)=25.13, P<0.001). Correspondingly, the SND ratio was significantly higher in the DRD3−/− than the WT mice (Figure 5b; U=0.50, P<0.01). Furthermore, the DRD3−/− mice showed no significant change in total juvenile investigatory activity during P1 or P2 showing the specificity of the behavioral change observed.
Comparison of the performance of dopamine D3 receptor deficient (DRD3−/−) to wild-type (WT) mice on social novelty discrimination (SND) in drug-free panels (a) and (b) and administration of the preferential dopamine D3 receptor antagonist, S33084. Bars show juvenile exploration (sec, mean±SEM, n=6) following a 30-min inter-trial interval (a, c). *P<0.05, **P<0.01 and ***P<0.001 from the novel juvenile at the same dose in the same treatment group (Bonferroni post hoc). The SND ratio (novel/familiar time) is shown in (b) and (d). **P<0.01 compared with vehicle. *P<0.05 compared with all other groups. Note that DRD3 knockout mice were able to distinguish between the familiar and novel juveniles after a 30-min inter-trial interval when WT mice were unable to discriminate.
After systemic injection with S33084 (0.63 mg/kg s.c.) WT mice spent significantly more time investigating the novel than the familiar juvenile (Figure 5c). DRD3−/− were able to discriminate the novel juvenile and S33084 treatment had no additional effect on this behavior. There was no significant main effect of genotype or drug injection on total investigation behavior during either P1 or P2 (Table 1), although there was a genotype × drug interaction in P2 (F(1,10)=4.89, P<0.05) and WT mice treated with S33084 had reduced total P2 compared with vehicle controls, they still preferentially explored the novel juvenile.
Effect of Dopamine D3 and D2 Antagonists on Delay-Dependent Impairment of NOR
The current study used NOR to distinguish the effects of dopamine D3 and D2 receptor antagonists on recognition memory impaired by using a 4-h inter-trial interval, a model of natural forgetting.
In dose-response studies with both the preferential dopamine D3 receptor antagonist, S33084, and the preferential dopamine D2 antagonist, L741,626, all treatment groups spent an equal amount of time exploring the two identical objects during the familiarization trial (T1, data not shown), confirming that rats had no innate spatial preference for either object location. Total object exploration during the familiarization trial was also unaffected by treatment with either drug (see Table 2). As expected in both studies rats treated with vehicle were unable to discriminate the novel from the familiar object in the choice trial following a 4-h ITI, spending an equivalent amount of time exploring each object (Figure 6a and c). Following treatment with S33084, repeated-measures ANOVA revealed a significant main effect of object (F(1,44)=26.19, P<0.001) and a significant object × treatment interaction (F(3,44)=4.04, P<0.05) in the pattern of object exploration during the choice trial (Figure 6a). S33084 caused a redistribution of object exploration; producing a dose-related and significant increase in exploration of the novel over the familiar object without altering total exploration in the choice trial. In confirmation of this increase in NOR, there was a concomitant increase in the d2 score (Figure 6b; F(3,44)=15.36, P<0.001), consistent with it being due to redistribution of exploration time without any change in total object exploration during the choice trial (Table 2). In contrast, after treatment with L741,626, there was no significant main effect of object or treatment, nor any object × treatment interaction (Figure 6c). Irrespective of the dose of L741,626 used rats were unable to discriminate the novel from the familiar object such that the discrimination ratio (d2) for all groups was unaltered and remained close to chance (Figure 6d).
Dose–response effect and interaction between the preferential dopamine D3 receptor antagonist, S33084, and the preferential dopamine D2 receptor antagonist, L741,626 on performance in the novel object recognition task, using a 4-h inter-trial interval in the rat. Data in (a, c, and e) show the actual time spent investigating the novel compared with the familiar object in the second choice trial (sec, mean±SEM, n=11–12). ***P<0.001 from the novel object at the same dose within the same treatment group (Bonferonni post hoc following ANOVA). Exploration times were also used to calculate the d2 score (see Materials and methods for definition) as an index of preferential object exploration in the choice trial and is shown (b, d, and f). (b) *P<0.05 from the novel object at the same dose. (f) **P<0.01 and ***P<0.001 compared with vehicle/vehicle group, ++P<0.01 compared with vehicle/S33084. Note that the D3 receptor antagonist was able to restore a time induced natural forgetting (a, b) in the novel object discrimination paradigm which was prevented by co-administration of the D2 receptor antagonist (e, f) while the latter had no effect on its own (c, d).
After the combined treatment of L741,626 (0.63 mg/kg) and S33084 (0.16 mg/kg), there was no significant effect of either drug (alone or in combination) on the total exploration of the objects during the familiarization trial (Table 2). One rat was excluded from the data due to not performing the task (spending <1 s attending the objects). Statistical analysis of object exploration during the choice trial revealed significant main effects of object (F(1,40)=25.81, P<0.001), an object × S33084 treatment interaction (F(1,40)=22.03, P<0.001), object × L741,626 treatment interaction (F(1,40)=6.09, P<0.05), and object × S33084 treatment × L741,626 treatment interaction (F(1,40)=8.15, P<0.01) (Figure 6e). Analysis of the d2 score (Figure 6f) revealed a significant main effect of S33084 (F(1,40)=25.34, P<0.001), but not L741,626 and a significant S33084 × L741,626 interaction (F(1,40)=5.77, P<0.05) with both vehicle/vehicle and L741,626/vehicle-treated groups being unable to discriminate the novel from the familiar object while, as with the previous experiment, S33084 restored discrimination. Although rats showed a significant degree of discrimination of the novel over familiar object after combined treatment of S33084 and L741,626 using the d2 score (P<0.01 compared with vehicle/vehicle) this was significantly lower than that of the S33084/vehicle group (P<0.01). Although there was a significant effect of L741,626 (F(1,40)=6.79, P<0.05) on total object exploration during the choice trial there was no effect of S33084 nor any S33084 × L741,626 interaction and no group had significantly different total exploration times from any other (Table 2).
Effect of Dopamine D2 Antagonist and Dopamine D3 Agonist on NOR with a Short Inter-Trial Interval
The effect of the preferential dopamine D2 antagonist, L741,626, and the dopamine D3 agonist, PD128,907, was examined on NOR using a short (2 min inter-trial interval) delay, so that control rats could readily discriminate the novel from the familiar object, and any amnesic properties of the compounds could be evaluated.
Neither L741,626 nor PD128,907 had any significant effect on the total exploration of both objects during the familiarization trial (Table 2) or on the distribution of exploration across both objects which were explored equally (data not shown). With both compounds, vehicle-treated rats were able to discriminate the novel from the familiar object (Figure 7a-d). Furthermore, in both cases there was a significant main effect of object (L741,626: F(1,44)=16.53, P<0.001; PD128,907: F(1,44)=24.31, P<0.001), and a significant object × treatment interaction (L741,626: F(3,44)=30.31, P<0.001; PD128,907: F(3,44)=6.87, P<0.01) during the second choice trial. Such that both L741,626 and PD128,907 caused a dose-related impairment in performance (d2 score: L741,626: F(3,44)=6.95, P<0.01, PD128,907: F(3,44)=6.10, P<0.01). The inability to distinguish the novel from the familiar object resulted from a redistribution of exploration time between the objects rather than a global reduction in exploration, as total object exploration during the choice trial was not significantly affected by either drug (Table 2).
Examination of the dose–response effects and interaction between the preferential dopamine D2 receptor antagonist, L741,626, and the dopamine D3 receptor agonist, PD128,907 on novel object recognition task, using a 2-min inter-trial interval in the rat. Data in (a, c, and e) show the time spent investigating the novel compared with the familiar object in the choice trial (sec, mean±SEM, n=12). **P<0.01 and ***P<0.001 from the novel object at the same dose within the same treatment group (Bonferonni post hoc). Panels (b, d, and f) show the derived d2 score (see Materials and methods for definition). (b, d) **P<0.01 compared with vehicle, ***P<0.01 compared with vehicle, (f) **P<0.01 compared with vehicle/vehicle group, ++P<0.01 compared with vehicle/PD128,907, Bonferonni post hoc following ANOVA. Both the D2 receptor antagonist (a, b) and the D3 receptor agonist (c, d) impaired object discrimination and the effect of the latter was prevented by pretreatment with a D3 receptor antagonist (e, f).
Treatment with the dopamine D3 receptor antagonist, S33084 (0.16 mg/kg), produced no significant enhancement in discrimination, which was not surprising since after a short inter-trial interval rats were already able to discriminate the novel object (Figure 7e and f). As previously seen, rats treated with PD128,907 (2.5 μg/kg) alone (after vehicle) were unable to discriminate between the objects in the choice trial. In contrast, rats treated with S33084 before PD128,907 retained the ability to discriminate the objects, as seen in Figure 7e where exploration of the novel is significantly higher than the familiar object except in the vehicle/PD128,907 treatment combination (P<0.001 repeated-measure ANOVA; object: F(1,44)=89.41, P<0.001; object × S33084 treatment interaction: F(1,44)=8.23, P<0.01; object × PD128,907 treatment interaction: F(1,44)=3.26, P=0.08, object × S33084 × PD128,907 treatment interaction: not significant). In agreement with the raw exploration data, the d2 score for the vehicle/PD128,907 group was significantly lower than all other drug combinations in that group (Figure 7f, P<0.01 compared with all others, two-way ANOVA; S33084 treatment: F(1,44)=7.59, P<0.01; PD128,907 treatment: not significant; S33084 × PD128,907 treatment interaction: F(1,44)=6.49, P<0.05). Neither drug treatment had any significant effect on the distribution of exploration of the two familiar objects during the familiarization trial, which were explored equally in all groups (data not shown). Neither drug significantly affected total object exploration during either the familiarization or choice trials, thus changes in object discrimination reflect a redistribution of exploration between the two objects (Table 2).
Effect of Bilateral Prefrontal Cortical or Striatal Microinjection of Dopamine D2 and D3 Receptor Antagonists on NOR
Bilateral microinjection of the dopamine D3 receptor antagonist, S33084, into the PFC had no significant effect on either the distribution of object exploration (data not shown) or total object exploration during the familiarization trial (Table 2) although the latter appeared to be slightly higher than in other experiments. After a 4-h inter trial-interval, vehicle-treated rats were unable to discriminate the novel and familiar objects (Figure 8a) during the choice trial. However, both doses of S33084 (0.63 and 2.5 μg/side) significantly increased novel object discrimination (P<0.05 and 0.01, respectively, compared with vehicle; Figure 8b). Repeated-measures ANOVA of the choice trial data showed a significant main effect of object (F(1,21)=27.92, P<0.001) and a significant object × treatment interaction (F(2,44)=5.53, P<0.05). Similarly, when expressed as the d2 score there was a main effect of treatment (F(2,21)=6.52, P<0.01) confirming that PFC microinjection of S33084 reversed a delay-induced impairment in object discrimination. S33084 treatment had no main effect on total choice trial object exploration showing that the improvement in novel object exploration resulted from a shift in attention away from the familiar object (Table 2).
Comparison of the effect of bilateral microinjection of the preferential dopamine D3 receptor antagonist, S33084 and the preferential dopamine D2 receptor antagonist, L741,626, into the rat prefrontal cortex and striatum on novel object recognition using a 4-h and 2-min inter-trial interval, respectively. Panels (a) and (c) show the time spent (mean±SEM, n=8–9) investigating the novel compared with the familiar object in the choice trial following drug injection into the prefrontal cortex. **P<0.01 and ***P<0.001 from the novel object at the same dose within the same treatment group (Bonferonni post hoc). Exploration times were used to calculate as the d2 score (see Materials and methods for definition) (b, d) and evaluate preferential object exploration as an index of recognition memory. *P<0.05, **P<0.01 and ***P<0.001 compared with vehicle following ANOVA. Panels (e) and (f) show the derived d2 score from the choice trial exploration times after injection into the striatum of S33084 using a 4-h inter-trial interval or L741,626 using a 2-min inter-trial interval, respectively. When injected into the prefrontal cortex the two antagonists had opposite effects on object discrimination such that the D3 receptor antagonist reversed natural forgetting (a, b) while the D2 antagonist impaired discrimination (c, d). In contrast, neither drug had any effect on novel object recognition when injected into the striatum (e, f).
Bilateral PFC microinjection of the dopamine D2 antagonist, L741,626, had no significant effect on the distribution of exploration of the two objects during the familiarization trial (data not shown) or on the total object exploration (Table 2) during either the familiarization or choice trials. By using a 2-min inter-trial as expected vehicle-treated rats spent more time exploring the novel than the familiar object during the choice trial (Figure 8c). Drug treatment significantly altered this pattern of object exploration (object: F(1,32)=37.52, P<0.001; object × treatment interaction: F(3,32)=13.81, P<0.001) producing a dose-dependent (0.63–5.0 μg/side) reduction in novel vs familiar object exploration. L741,626 significantly reduced the d2 ratio at all doses compared with that of vehicle controls (Figure 8d, treatment: F(3,32)=17.56, P<0.001) consistent with it impairing visual-recognition memory. In contrast, microinjections of S33084 and L741,626 into the striatum had no effect on NOR performance (Figure 8e and f). With a long inter-trial interval, microinjections of S33084 (2.5 μg/side) into the striatum did not restore performance and the d2 score remained unaltered (Figure 8e). Both vehicle- and S33084-treated animals explored the novel and familiar object to a similar extent during the choice trial (data not shown). With a 2-min inter-trial interval, microinjections of L741,626 (2.5 μg/side) into the striatum caused no impairment of novel object discrimination (Figure 8f). Rats given intra-striatal L741,626 preferentially explored the novel compared with the familiar object (data not shown; object: F(1,17)=54.32, P<0.001), but this was not affected by drug (object × treatment interaction, not significant). Microinjection of S33084 or L741,626 into the striatum had no effect on total object exploration during either the familiarization or choice trials (Table 2).
DISCUSSION
The current study is the first systematic comparison of the contrasting role of dopamine D3 and D2 receptors in two rodent models of learning and memory; the SND and NOR tests using selective ligands and a D3 knockout mouse. The results support the proposal that blockade of dopamine D3 receptors enhances cognitive function, while activation of the dopamine D3 receptor with agonists or blockade of dopamine D2 receptors impairs learning and memory in these paradigms. Furthermore, this is the first study using microinjection studies to identify a potentially important brain site of the differential actions of dopamine D2 and D3 receptor ligands in two complimentary cognitive behavioral tasks. The data suggest that dopamine D2 and D3 receptors located in the PFC have opposing roles in modulation performance of both SND and NOR while the striatum has a much smaller or negligible role in these procedures.
Effects of Dopamine Antagonists on SND
The SND paradigm utilizes an innate behavioral response to investigate novel conspecifics as an index of the ability of rodents to discriminate a previously investigated familiar from a novel juvenile rat or mouse (Engelmann et al, 1995; Ferguson et al, 2002). The primary salient cue for social discrimination is olfactory and the task examines short-term memory involving a strong attentional component (Ferguson et al, 2002). SND is disrupted by extending the inter-trial interval, other parametric modification (reducing the time available to investigate the juvenile during P1), acute injections of phencyclidine (PCP) and neonatal treatment with PCP (Engelmann et al, 1995; Terranova et al, 2005). SND is thought to be a test of social recognition, in a separate cognitive domain from the visual learning and memory involved in NOR (Young et al, 2009) described below. SND involves the evaluation and response to social cues, the accessory olfactory bulb and the neuropeptides oxytocin and vasopressin which enhance acquisition and consolidation, respectively (Insel and Fernald, 2004); none of which are involved in NOR. Indeed, oxytocin knockout mice show specific deficits in SND without any alteration in non-social memory (Ferguson et al, 2000; Goodson and Thompson, 2010), confirming that SND and NOR map to different cognitive domains. In the delay-induced deficit protocol used herein, the delay alone (30 min) is insufficient to induce natural forgetting of the juvenile presented in P1, since the adult rat can successfully discriminate the novel from the familiar juvenile if their movement is restricted in mesh cages on opposite sides of the arena in P2 (Terranova et al, 2005). Thus, the free movement of the juvenile rats necessitates investment of sustained and selective attention as well as effective working memory (Engelmann et al, 1995; Terranova et al, 2005), which are both essential to enable performance of the SND task.
In the current study, selective antagonism of the dopamine D3 receptor by S33084 caused a progressive improvement in SND with increasing doses when the inter-trial interval was sufficiently long to reduce discrimination from maximal. In contrast, both the dopamine D3 agonist, PD128,907, and the D2 antagonist, L741,626, impaired SND when this was maximal following a short inter-trial delay. Consistent with the proposal that dopamine D2 and D3 receptors have opposing modulatory effects on learning and memory (see Introduction), the improvement in SND seen with S33084 was also reversed by prior treatment with L741,626. Microdialysis studies show that systemic administration the dopamine D2 and D3 compounds utilized herein alter neurotransmitter release for at least 2 h (Millan et al, 2000b; Millan et al, 2004), so active CNS concentrations will be present during both acquisition and consolidation. However, S33084 (0.63 mg/kg) produces an equivalent reversal of the delay-induced impairment when administered 1 min after P1 (data not shown), suggesting that the effects of D3 antagonists are unlikely to be the result from improved acquisition, but rather augmentation of the rat's exploitation of information acquired during P2. Although few compounds with selectivity for individual dopamine receptors have been used in this paradigm, the enhanced SND produced by S33084 is comparable to that seen with the dopamine D3 receptor antagonist, S33138 (Millan et al, 2010). Further, in light of the data presented herein, it is likely that the reversal of SND disruption by the mixed dopamine D2 receptor antagonist and 5-HT1A receptor agonist, SSR181507, is due to its activation of 5-HT1A receptors (Terranova et al, 2005). It is, however, noteworthy that both the atypical antipsychotics, clozapine and amisulpiride, and to a lesser extent (at one dose) the typical antipsychotic, haloperidol, reverse a time delay-induced SND deficit (Terranova et al, 2005), while these drugs have limited efficacy against the cognitive deficits of schizophrenia (Keefe et al, 2007), so any translational relevance of such findings needs to be made with caution.
Transgenic mice lacking the dopamine D3 receptor (DRD3−/−) showed a greater spontaneous discrimination of a novel juvenile than WT controls in SND in the current study. Furthermore, while S33084 improved SND performance in WT mice it had no effect on behavior in DRD3−/− mice. Consistent with having an elevated basal social memory, DRD3−/− mice show enhanced performance in a step-through passive-avoidance test (Micale et al, 2010) and set-shifting performance in an attentional set-shifting task accompanied by increased c-fos expression in the anterior cingulate, prelimbic, and infralimbic cortices compared with WT (Glickstein et al, 2005). In contrast, dopamine D2 receptor knockout mice are impaired in the first compound discrimination of the attentional set-shifting task and have significantly lower c-fos expression compared with WT (Glickstein et al, 2005). Interestingly in a delayed alternation spatial working memory task both DRD2−/− and DRD3−/− mutant mice were impaired compared with WT (Glickstein et al, 2002). However, this response was fully (DRD2−/−) and partially (DRD3−/−) rescued by repeated dopamine D1 receptor agonist treatment, which caused a parallel increase in c-fos expression in PFC neurons, consistent with PFC dopamine D1 receptor activation restoring working memory deficits in both these mutants. Collectively, these data suggest that dopamine D2 and D3 receptors may have distinctive roles in particular learning and memory tasks reflecting their distinct pattern of neuronal distribution.
The role of dopamine D3 receptors in cognitive functions has recently been highlighted in a model of neurofibromatosis, a genetic developmental disorder associated with tumor predisposition and cognitive deficits. Mice carrying a heterozygous null mutation of the Nf1 gene (NF1+/−) associated with neurofibromatosis exhibit spatial working memory deficits (Costa et al, 2002). Network analysis of gene expression in these mutant mice suggests that cognitive deficits may relate to alterations in the trafficking of complexes involving neurofibromin (NF1), amyloid precursor protein (APP), and the dopamine D3 receptor (Donarum et al, 2006). Furthermore, both levels of APP protein and mRNA were significantly increased while NF1 levels were decreased compared with WT mice in dopamine D3 knockout subjects (Castorina et al, 2011). Hence, these KO studies indicate a potentially broader relevance of dopamine D3 receptors in cognition and CNS disorders.
Effects of Dopamine Antagonists on Recognition Memory
NOR is a form of visual-recognition memory dependent on spontaneous innate preference of rats to investigate novel objects removing the requirement for training, motivational food reward, or aversive stimuli (Ennaceur and Delacour, 1988). The task has translational relevance to the visual-recognition memory impairments seen in schizophrenia (Young et al, 2009). NOR is altered by increasing the inter-trial interval (King et al, 2004; Sutcliffe et al, 2007), pretreatment with NMDA receptor antagonists such as PCP or MK-801 (Grayson et al, 2007), muscarinic receptor antagonists, such as scopolamine (Woolley et al, 2003), and neurodevelopmental interventions such as isolation rearing of pups from weaning (Bianchi et al, 2006; Fone and Porkess, 2008; Jones et al, 2011). Collectively, these data suggest that NOR is regulated by both cholinergic and glutamatergic mechanisms. Consistent with the current report one previous study showed that dopamine D2/D3 receptor antagonist, raclopride, impairs NOR (Woolley et al, 2003). The present study extends these findings showing that blockade of the dopamine D3 receptor enhances, while D3 receptor activation or D2 receptor blockade impairs NOR, consistent with SND studies. Although comparable pharmacological effects are seen in both NOR and SND tasks, the former appears to be more sensitive, requiring ∼4-fold lower doses to achieve significant mnemonic or amnesic activity than required in the SND task. However, as discussed below, when S33084 was administered into the PFC it showed an apparently equivalent dose effect on both behaviors suggesting that the different systemic potency reflects divergent post-dopaminergic anatomical pathways mediating the two behavioral changes. We have also characterized other drug classes, including mGluR, 5-HT6 and NMDA ligands across the two procedures and the difference in potency is inconsistent but not a unique feature of drugs interacting with the dopamine D3 receptor. Previously, we demonstrated another, less selective, dopamine D3 antagonist, S33138, reverses delay-dependent impairments in NOR in the rat (Millan et al, 2010). Furthermore, both S33138 and S33084 reverse a social isolation rearing deficit in NOR (Watson et al, 2011), and this study shows the dopamine D3 agonist, PD128,907, impairs object recognition in group-housed controls. In the marmoset, the dopamine D3 receptor agonist, 7-OH-DPAT, impaired visual object discrimination performance (Smith et al, 1999) and passive-avoidance learning in mice (Ukai et al, 1997), supporting the amnesic properties of D3 receptor activation across species. Of note, S33084 reverses the PD128,907-induced impairment in NOR, presumably through common receptor antagonism. While blockade of D3 receptors enhanced NOR, antagonism of D2 receptors with L741,626 impaired NOR after short inter-trial delays. Furthermore, L741,626 attenuated the enhancement of NOR produced by S33084. It also impaired NOR in group-housed rats using a 2-h inter-trial interval (Watson et al, 2011) and blockade of D2 sites presumably explains why the mixed D2/D3 antagonists, raclopride and eticlopride, both impaired recognition memory in rats (Besheer et al, 1999; Woolley et al, 2003). Of note, the marked effects of the drugs used (or genetic alteration) on discrimination in NOR (and SND) cannot be explained by any change in total T2 (or P2, respectively) and are consistent with these being due to changes in learning and memory rather than altered motivation to perform the task. As drugs were administered before the first habituation trial in the present work, the stage(s) of learning and memory (encoding, consolidation or retrieval) affected by D3 and D2 receptors warrants evaluation in future studies.
CNS Sites of Action of Dopamine D3 and D2 Receptors Ligands
To attempt to identify the CNS location of dopamine D3 and D2 receptors involved in the current cognitive effects, discrete microinjection studies were performed. SND was enhanced by bilateral injections of the D3 receptor antagonist, S33084, into the PFC while injection into the striatum had no effect. These results support previous studies in the social recognition task where S33084 reversed delay-induced impairments when injected into PFC but not into the striatum or nucleus accumbens (Loiseau and Millan, 2009). In contrast, bilateral microinjection of the preferential dopamine D2 receptor antagonist, L741,626, into the PFC did not reverse the impairment in social recognition. Although the neural pathways underlying SND have not been well defined, the behavior relies on chemosensory cues to form an ‘olfactory signature’ for the familiar juvenile, and areas known to be involved in social cognition include the piriform, entorhinal, and perirhinal cortices and the amygdaloid complex (Davis, 2004; Ferguson et al, 2002). These areas contain low levels of dopamine D3 receptors but further work would be needed to specifically exclude these regions in the effect of dopamine D3 antagonists on SND.
Low doses of psychostimulants such as methylphenidate, which improve attention in the rodent, elevate PFC dopamine release measured by microdialysis, consistent with this being involved in attention (Berridge et al, 2006). PET studies in baboons show dopamine D3 receptor-mediated regional cerebral blood flow responses are restricted to prefrontal and limbic cortices (Black et al, 2002), and performance in the PFC-dependent attentional set-shifting task is improved in dopamine D3−/− receptor mice (Glickstein et al, 2005). A distributed network of neurons in the prefrontal and parietal cortex is involved in the top-down regulation of attention, regulating eye movement and biasing sensory processing in favor of behaviorally relevant information, so improving saliency (Noudoost et al, 2010; Rossi et al, 2009). Recent human fMRI studies show that disruption of PFC activation (mimicking the disruption seen of GABA-driven neural synchrony, attention and working memory in schizophrenia; Gonzalez-Burgos et al, 2010) diminishes selective attention and subsequent recognition memory consistent with this top-down regulation of visual processing and working memory (Rauss et al, 2011; Zanto et al, 2011). Collectively, these studies in mice, rats and primates support the importance of PFC dopamine D3 receptor activation in the control of cognitive processes involving attentional mechanisms. In a repeated amphetamine-induced model of psychosis and cognitive impairment, sustained attention in an operant task was accompanied by decreased PFC acetylcholine release (Sarter et al, 2009). It is well established that the PFC dopamine D3 receptors modulate cholinergic neurotransmission (Lacroix et al, 2003; Millan et al, 2007) consistent with this being a potential mechanism for the observed cognitive effects seen in this study. In addition, there is evidence that dopamine D3 receptors are located on astrocytes (Choi et al, 2006) where they may regulate release of D-serine which in turn activates NMDA receptors and could also enhance NOR and SND (Fossat et al, 2011).
Discrete bilateral microinjections of S33084 into the PFC caused a dose-related reversal of delay-induced impairment of NOR, while bilateral microinjection of the L741,626 into the same region impaired NOR. Of note, systemic administration of the dopamine D1 agonist SKF 8129, which improves NOR memory after a 4-h delay (Hotte et al, 2005), increases phosphorylation of CREB and dopamine and cAMP-regulated phosphoprotein 32 kDa (DARPP-32) two substrates linked to the D1 transduction system in the PFC (Hotte et al, 2006). Additionally, bilateral microinjection of the dopamine D1 receptor antagonist, SCH23390, into the PFC of mice altered recognition memory and increased ERK1/2 phosphorylation therein (Nagai et al, 2007). Facilitation of NMDA receptor-dependent neurotransmission by the selective deletion of forebrain glycine transporter 1 (GlyT1) also enhances object recognition (Singer et al, 2007) and social cognition (Shimazaki et al, 2010). Collectively, this suggest that although the PFC may not be critical for task performance, it exerts a powerful modulatory effect on NOR. Lesion and pharmacological studies have established the importance of medial temporal lobe structures such as the perirhinal and entorhinal cortices in the mediation of recognition memory (Abe et al, 2004; Aggleton et al, 1997; Ennaceur and Aggleton, 1997), though the role of the hippocampus is more contentious (Ainge et al, 2006; Gould et al, 2002; Hammond et al, 2004). As there is little dopamine D3 receptor expression in the hippocampus this is unlikely to be an important site of action in the current study but further studies would be required to exclude this.
The apparent amnesic property of dopamine D2 receptor blockade has significant implications for the treatment of schizophrenia. Antagonism at dopamine D2 receptors is a common pharmacological mechanism of existing antipsychotic drugs which do not effectively treat the cognitive deficits seen in schizophrenia (Keefe et al, 2007). One strategy to improve cognitive therapy with antipsychotic drugs is to develop optimized dopamine D3 and D2 antagonists like S33138 (Joyce and Millan, 2005). However, the potential therapeutic benefit of targeting dopamine D3 receptors is not restricted to the treatment of cognitive dysfunction in schizophrenia (Millan and Brocco, 2008) but also in other common CNS disorders such as Parkinson's disease, Alzheimer's disease and as mentioned above neurofibromatosis and autism-related disorders.
CONCLUSIONS
In conclusion, the current data show that selective antagonism of dopamine D3 receptor reverses delay-induced impairment of both visual-recognition and SND memory. In contrast, both activation of dopamine D3 receptors and antagonism of dopamine D2 receptors impair NOR and SND performance. Furthermore, supporting previous work, these cognitive effects are mediated, at least in part, by the PFC which likely exerts its actions by top-down control of cognitive (attentional and other) processes integrated in other regions processing social-olfactory (such as amygdala and lateral septum) and visual-recognition (perirhinal and entorhinal cortex) cues. These structures have relatively few D3 (and D2) receptors, but any role in the control of SND and NOR by dopamine D3 and D2 receptor ligands remains to be examined. It would be worthwhile to compare the role of dopamine D3 and D2 receptors in the regulation of other cognitive tasks regulated by the PFC such as attentional set-shifting and other procedures interrogating executive function to further delineate the importance of this structure in the changes in learning and memory observed and to confirm the differential involvement of dopamine D2 and D3 receptors by evaluation of behavior in D2 mutant mice.
References
Abe H, Ishida Y, Iwasaki T (2004). Perirhinal N-methyl-D-aspartate and muscarinic systems participate in object recognition in rats. Neurosci Lett 356: 191–194.
Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH et al (1996). A targeted mutation of the D-3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci USA 93: 1945–1949.
Aggleton JP, Keen S, Warburton EC, Bussey TJ (1997). Extensive cytotoxic lesions involving both the rhinal cortices and area TE impair recognition but spare spatial alternation in the rat. Brain Res Bull 43: 279–287.
Ainge JA, Heron-Maxwell C, Theofilas P, Wright P, de Hoz L, Wood ER (2006). The role of the hippocampus in object recognition in rats: examination of the influence of task parameters and lesion size. Behav Brain Res 167: 183–195.
Baluch F, Itti L (2011). Mechanisms of top-down attention. Trends Neurosci 34: 210–224.
Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AFT, Kelley AE, Schmeichel B et al (2006). Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry 60: 1111–1120.
Besheer J, Jensen HC, Bevins RA (1999). Dopamine antagonism in a novel-object recognition and a novel-object place conditioning preparation with rats. Behav Brain Res 103: 35–44.
Bianchi M, Fone KCF, Azmi N, Heidbreder CA, Hagan JJ, Marsden CA (2006). Isolation rearing induces recognition memory deficits accompanied by cytoskeletal alterations in rat hippocampus. Eur J Neurosci 24: 2894–2902.
Bianchi M, Shah AJ, Fone KCF, Atkins AR, Dawson LA, Heidbreder CA et al (2009). Fluoxetine administration modulates the cytoskeletal microtubular system in the rat hippocampus. Synapse 63: 359–364.
Black KJ, Hershey T, Koller JM, Videen TO, Mintun MA, Price JL et al (2002). A possible substrate for dopamine-related changes in mood and behavior: prefrontal and limbic effects of a D3-preferring dopamine agonist. Proc Natl Acad Sci USA 99: 17113–17118.
Boeckler F, Gmeiner P (2006). The structural evolution of dopamine D-3 receptor ligands: structure-activity relationships and selected neuropharmacological aspects. Pharmacol Ther 112: 281–333.
Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC (1991). Localization of dopamine D3 receptor messenger RNA in the rat brain using in situ hybridization histochemistry - comparison with dopamine D2 receptor messenger. Brain Res 564: 203–219.
Castorina A, Leggio GM, Giunta S, Magro G, Scapagnini G, Drago F et al (2011). Neurofibromin and amyloid precursor protein expression in dopamine d3 receptor knock-out mice brains. Neurochem Res 36: 426–434.
Choi JK, Chen YI, Hamel E, Jenkins BG (2006). Brain hemodynamic changes mediated by dopamine receptors: Role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage 30: 700–712.
Chudasama Y, Robbins TW (2004). Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology 29: 1628–1636.
Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M et al (2002). Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature 415: 526–530.
Cussac D, Newman-Tancredi A, Sezgin L, Millan MJ (2000). The novel antagonist, S33084, and GR218,231 interact selectively with cloned and native, rat dopamine D(3) receptors as compared with native, rat dopamine D(2) receptors. Eur J Pharmacol 394: 47–50.
Davis RL (2004). Olfactory learning. Neuron 44: 31–48.
DeSteno DA, Schmauss C (2009). A role for dopamine D2 receptors in reversal learning. Neuroscience 162: 118–127.
Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC et al (2000). Dopamine D-3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci 20: 8677–8684.
Donarum EA, Halperin RF, Stephan DA, Narayanan V (2006). Cognitive dysfunction in NF1 knock-out mice may result from altered vesicular trafficking of APP/DRD3 complex. BMC Neurosci 7: 22.
Ehrlich K, Gotz A, Bollinger S, Tschammer N, Bettinetti L, Harterich S et al (2009). Dopamine D-2, D-3, and D-4 selective phenylpiperazines as molecular probes to explore the origins of subtype specific receptor binding. J Med Chem 52: 4923–4935.
Engelmann M, Wotjak CT, Landgraf R (1995). Social discrimination procedure - an alternative method to investigate juvenile recognition abilities in rats. Physiol Behav 58: 315–321.
Ennaceur A, Aggleton JP (1997). The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behav Brain Res 88: 181–193.
Ennaceur A, Delacour J (1988). A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31: 47–59.
Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT (2000). Social amnesia in mice lacking the oxytocin gene. Nat Genet 25: 284–288.
Ferguson JN, Young LJ, Insel TR (2002). The neuroendocrine basis of social recognition. Front Neuroendocrinol 23: 200–224.
Fone KCF, Porkess MV (2008). Behavioural and neurochemical effects of post-weaning social isolation in rodents-Relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev 32: 1087–1102.
Fossat P, Turpin FR, Sacchi S, Dulong J, Rivet JM, Polleioni L et al (2011). Glial D-serine gates NMDA receptors at excitatory synapses in prefrontal cortex. Cereb Cortex (in press).
Glickstein SB, Desteno DA, Hof PR, Schmauss C (2005). Mice lacking dopamine D2 and D3 receptors exhibit differential activation of prefrontal cortical neurons during tasks requiring attention. Cereb Cortex 15: 1016–1024.
Glickstein SB, Hof PR, Schmauss C (2002). Mice lacking dopamine D2 and D3 receptors have spatial working memory deficits. J Neurosci 22: 5619–5629.
Gonzalez-Burgos G, Hashimoto T, Lewis DA (2010). Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep 12: 335–344.
Goodson JL, Thompson RR (2010). Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr Opin Neurobiol 20: 784–794.
Gould TJ, Rowe WB, Heman KL, Mesches MH, Young DA, Rose GM et al (2002). Effects of hippocampal lesions on patterned motor learning in the rat. Brain Res Bull 58: 581–586.
Grayson B, Idris NF, Neill JC (2007). Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behav Brain Res 184: 31–38.
Grundt P, Husband SLJ, Luedtke RR, Taylor M, Newman AH (2007). Analogues of the dopamine D2 receptor antagonist L741,626: Binding, function, and SAR. Bioorg Med Chem Lett 17: 745–749.
Hammond RS, Tull LE, Stackman RW (2004). On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem 82: 26–34.
Heidbreder CA, Newman AH (2010). Current perspectives on selective dopamine D-3 receptor antagonists as pharmacotherapeutics for addictions and related disorders. Addict Rev 2 1187: 4–34.
Herroelen L, Debacker JP, Wilczak N, Flamez A, Vauquelin G, Dekeyser J (1994). Autoradiographic distribution of D-3-type dopamine receptors in human brain using [H-3] 7-Hydroxy-N,N-di-N-propyl-2-aminotetralin. Brain Res 648: 222–228.
Holmes A, Lachowicz JE, Sibley DR (2004). Phenotypic analysis of dopamine receptor knockout mice; recent insights into the functional specificity of dopamine receptor subtypes. Neuropharmacology 47: 1117–1134.
Hotte M, Naudon L, Jay TM (2005). Modulation of recognition and temporal order memory retrieval by dopamine D1 receptor in rats. Neurobiol Learn Mem 84: 85–92.
Hotte M, Thuault S, Lachaise F, Dineley KT, Hemmings HC, Nairn AC et al (2006). D1 receptor modulation of memory retrieval performance is associated with changes in pCREB and pDARPP-32 in rat prefrontal cortex. Behav Brain Res 171: 127–133.
Insel TR, Fernald RD (2004). How the brain processes social information: searching for the social brain. Ann Rev Neurosci 27: 697–722.
Jones CA, Brown AM, Auer DP, Fone KC (2011). The mGluR2/3 agonist LY379268 reverses post-weaning social isolation-induced recognition memory deficits in the rat. Psychopharmacology (Berl) 214: 269–283.
Joyce JN (2001). Dopamine D3 receptor as a therapeutic target for antipsychotic and antiparkinsonian drugs. Pharmacol Ther 90: 231–259.
Joyce JN, Millan MJ (2005). Dopamine D-3 receptor antagonists as therapeutic agents. Drug Discov Today 10: 917–925.
Keefe RSE, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM et al (2007). Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch Gen Psychiatry 64: 633–647.
King MV, Seeman P, Marsden CA, Fone KCF (2009). Increased dopamine D-2(high) receptors in rats reared in social isolation. Synapse 63: 476–483.
King MV, Sleight AJ, Woolley ML, Topham IA, Marsden CA, Fone KC (2004). 5-HT6 receptor antagonists reverse delay-dependent deficits in novel object discrimination by enhancing consolidation--an effect sensitive to NMDA receptor antagonism. Neuropharmacology 47: 195–204.
Klinkenberg I, Sambeth A, Blokland A (2011). Acetylcholine and attention. Behav Brain Res 221: 430–442.
Kulagowski JJ, Broughton HB, Curtis NR, Mawer IM, Ridgill MP, Baker R et al (1996). 3-[[4-(4-chlorophenyl)piperazin-1-yl]methyl]-1H-pyrrolo[2,3-b]pyridine: An antagonist with high affinity and selectivity for the human dopamine D-4 receptor. J Med Chem 39: 1941–1942.
Lacroix LP, Hows MEP, Shah AJ, Hagan JJ, Heidbreder CA (2003). Selective antagonism at dopamine D-3 receptors enhances monoaminergic and cholinergic neurotransmission in the rat anterior cingulate cortex. Neuropsychopharmacology 28: 839–849.
Lapiz MD, Mateo Y, Parker T, Marsden C (2000). Effects of noradrenaline depletion in the brain on response on novelty in isolation-reared rats. Psychopharmacology (Berl) 152: 312–320.
Laszy J, Laszlovszky I, Gyertyan I (2005). Dopamine D3 receptor antagonists improve the learning performance in memory-impaired rats. Psychopharmacology (Berl) 179: 567–575.
Li Y-C, Kellendonk C, Simpson EH, Kandel ER, Gao W-J (2011). D2 receptor overexpression in the striatum leads to a deficit in inhibitory transmission and dopamine sensitivity in mouse prefrontal cortex. Proc Natl Acad Sci USA 108: 12107–12112.
Loiseau F, Millan MJ (2009). Blockade of dopamine D-3 receptors in frontal cortex, but not in sub-cortical structures, enhances social recognition in rats: Similar actions of D-1 receptor agonists, but not of D-2 antagonists. Eur Neuropsychopharmacol 19: 23–33.
Meador-Woodruff JH, Damask SP, Wang J, Haroutunian V, Davis KL, Watson SJ (1996). Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology 15: 17–29.
Micale V, Cristino L, Tamburella A, Petrosino S, Leggio GM, Di Marzo V et al (2010). Enhanced cognitive performance of dopamine D3 receptor ″knock-out″ mice in the step-through passive-avoidance test: Assessing the role of the endocannabinoid/endovanilloid systems. Pharmacol Res 61: 531–536.
Millan MJ, Brocco M (2008). Cognitive impairment in schizophrenia: a review of developmental and genetic models, and pro-cognitive profile of the optimised D(3) > D(2) antagonist, S33138. Therapie 63: 187–229.
Millan MJ, Buccafusco JJ, Loiseau F, Watson DJ, Decamp E, Fone KC et al (2010). The dopamine D(3) receptor antagonist, S33138, counters cognitive impairment in a range of rodent and primate procedures. Int J Neuropsychopharmacol 13: 1035–1051.
Millan MJ, Dekeyne A, Rivet JM, Dubuffet T, Lavielle G, Brocco M (2000a). S33084, a novel, potent, selective, and competitive antagonist at dopamine D-3-receptors: II. Functional and behavioral profile compared with GR 218, 231 and L741, 626. J Pharmacol Exp Ther 293: 1063–1073.
Millan MJ, Di Cara B, Dekeyne A, Panayi F, De Groote L, Sicard D et al (2007). Selective blockade of dopamine D(3) versus D(2) receptors enhances frontocortical cholinergic transmission and social memory in rats: a parallel neurochemical and behavioural analysis. J Neurochem 100: 1047–1061.
Millan MJ, Gobert A, Newman-Tancredi A, Lejeune F, Cussac D, Rivet JM et al (2000b). S33084, a novel, potent, selective, and competitive antagonist at dopamine D-3-receptors: I. Receptorial, electrophysiological and neurochemical profile compared with GR 218, 231 and L741,626. J Pharmacol Exp Ther 293: 1048–1062.
Millan MJ, Mannoury la Cour C, Novi F, Maggio R, Audinot V, Newman-Tancredi A et al (2008). S33138 [N-[4-[2-(3aS,9bR)-8-cyano-1,3a,4,9b-tetrahydro[1]-benzopyrano[3,4-c]pyrr ol-2(3H)-yl]-ethyl]phenylacetamide], a preferential dopamine D3 versus D2 receptor antagonist and potential antipsychotic agent: I. Receptor-binding profile and functional actions at G-protein-coupled receptors. J Pharmacol Exp Ther 324: 587–599.
Millan MJ, Seguin L, Gobert A, Cussac D, Brocco M (2004). The role of dopamine D-3 compared with D-2 receptors in the control of locomotor activity: a combined behavioural and neurochemical analysis with novel, selective antagonists in rats. Psychopharmacology 174: 341–357.
Nagai T, Takuma K, Kamei H, Ito Y, Nakamichi N, Ibi D et al (2007). Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn Mem 14: 117–125.
Noudoost B, Chang MH, Steinmetz NA, Moore T (2010). Top-down control of visual attention. Curr Opin Neurobiol 20: 183–190.
Paxinos G, Watson C (1997). The Rat Brain in Stereotaxic Coordinates. Compact 3rd edn, Academic Press: San Diego, London. xxxiii, [78] p. of plates.
Pugsley TA, Davis MD, Akunne HC, MacKenzie RG, Shih YH, Damsma G et al (1995). Neurochemical and functional characterization of the preferentially selective dopamine D3 agonist PD 128907. J Pharmacol Exp Ther 275: 1355–1366.
Rabiner EA, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R et al (2009). In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: studies in non-human primates and transgenic mice. Synapse 63: 782–793.
Rauss K, Schwartz S, Pourtois G (2011). Top-down effects on early visual processing in humans: a predictive coding framework. Neurosci Biobehav Rev 35: 1237–1253.
Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY et al (2000). Pharmacological actions of a novel, high-affinity, and selective human dopamine D(3) receptor antagonist, SB-277011-A. J Pharmacol Exp Ther 294: 1154–1165.
Rossi AF, Pessoa L, Desimone R, Ungerleider LG (2009). The prefrontal cortex and the executive control of attention. Exp Brain Res 192: 489–497.
Sarter M, Martinez V, Kozak R (2009). A neurocognitive animal model dissociating between acute illness and remission periods of schizophrenia. Psychopharmacology 202: 237–258.
Shimazaki T, Kaku A, Chaki S (2010). d-Serine and a glycine transporter-1 inhibitor enhance social memory in rats. Psychopharmacology 209: 263–270.
Singer P, Boison D, Mohler H, Feldon J, Yee BK (2007). Enhanced recognition memory following glycine transporter 1 deletion in forebrain neurons. Behav Neurosci 121: 815–825.
Smith AG, Neill JC, Costall B (1999). The dopamine D3/D2 receptor agonist 7-OH-DPAT induces cognitive impairment in the marmoset. Pharmacol Biochem Behav 63: 201–211.
Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E et al (2006). The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets 5: 25–43.
Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC (1990). Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 347: 146–151.
Sutcliffe JS, Marshall KM, Neill JC (2007). Influence of gender on working and spatial memory in the novel object recognition task in the rat. Behav Brain Res 177: 117–125.
Terranova JP, Chabot C, Barnouin MC, Perrault G, Depoortere R, Griebel G et al (2005). SSR181507, a dopamine D(2) receptor antagonist and 5-HT(1A) receptor agonist, alleviates disturbances of novelty discrimination in a social context in rats, a putative model of selective attention deficit. Psychopharmacology (Berl) 181: 134–144.
Ukai M, Tanaka T, Kameyama T (1997). Effects of the dopamine D3 receptor agonist, R(+)-7-hydroxy-N,N-di-n-propyl-2-aminotetralin, on memory processes in mice. Eur J Pharmacol 324: 147–151.
Ventura R, Pascucci T, Catania MV, Musumeci SA, Puglisi-Allegra S (2004). Object recognition impairment in Fmr1 knockout mice is reversed by amphetamine: involvement of dopamine in the medial prefrontal cortex. Behav Pharmacol 15: 433–442.
Waddington JL, O′Tuathaigh C, O′Sullivan G, Tomiyama K, Koshikawa N, Croke DT (2005). Phenotypic studies on dopamine receptor subtype and associated signal transduction mutants: insights and challenges from 10 years at the psychopharmacology-molecular biology interface. Psychopharmacology 181: 611–638.
Watson DJ, Marsden CA, Millan MJ, Fone KC (2011). Blockade of dopamine D3 but not D2 receptors reverses the novel object discrimination impairment produced by post-weaning social isolation: implications for schizophrenia and its treatment. Int J Neuropsychopharmacol. doi:10/S1461145711000435.
Woolley ML, Marsden CA, Sleight AJ, Fone KCF (2003). Reversal of a cholinergic-induced deficit in a rodent model of recognition memory by the selective 5-HT6 receptor antagonist, Ro 04–6790. Psychopharmacology 170: 358–367.
Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA (2009). Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther 122: 150–202.
Zanto TP, Rubens MT, Thangavel A, Gazzaley A (2011). Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci 14: 656–661.
Acknowledgements
We thank Miss Stacey Knapp, Miss Clare Spicer, and Mr Ian Topham for their technical assistance and Institut de Recherches Servier for funding this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
David JG Watson, Florence Loiseau, Mark J Millan, Charles A Marsden, and Kevin CF Fone declare that, except for income received from their primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. The contribution to this work made by David JG Watson, Charles A Marsden, and Kevin CF Fone was financially supported by Servier.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Watson, D., Loiseau, F., Ingallinesi, M. et al. Selective Blockade of Dopamine D3 Receptors Enhances while D2 Receptor Antagonism Impairs Social Novelty Discrimination and Novel Object Recognition in Rats: A Key Role for the Prefrontal Cortex. Neuropsychopharmacol 37, 770–786 (2012). https://doi.org/10.1038/npp.2011.254
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2011.254
Keywords
This article is cited by
-
Reduction in the activity of VTA/SNc dopaminergic neurons underlies aging-related decline in novelty seeking
Communications Biology (2023)
-
Insights into the Involvement and Therapeutic Target Potential of the Dopamine System in the Posttraumatic Stress Disorder
Molecular Neurobiology (2023)
-
Cerebellar dopamine D2 receptors regulate social behaviors
Nature Neuroscience (2022)
-
Garcinia cambogia extract alters anxiety, sociability, and dopamine turnover in male Swiss albino mice
SN Applied Sciences (2022)
-
Prevention of age-associated neuronal hyperexcitability with improved learning and attention upon knockout or antagonism of LPAR2
Cellular and Molecular Life Sciences (2021)