Abstract
The non-competitive NMDA receptor (NMDA-R) antagonist phencyclidine (PCP)—used as a pharmacological model of schizophrenia—disrupts prefrontal cortex (PFC) activity. PCP markedly increased the discharge rate of pyramidal neurons and reduced slow cortical oscillations (SCO; 0.15–4 Hz) in rat PFC. Both effects were reversed by classical (haloperidol) and atypical (clozapine) antipsychotic drugs. Here we extended these observations to mice brain and examined the potential involvement of 5-HT2A and 5-HT1A receptors (5-HT2AR and 5-HT1AR, respectively) in the reversal by clozapine of PCP actions. Clozapine shows high in vitro affinity for 5-HT2AR and behaves as partial agonist in vivo at 5-HT1AR. We used wild-type (WT) mice and 5-HT1AR and 5-HT2AR knockout mice of the same background (C57BL/6) (KO-1A and KO-2A, respectively). Local field potentials (LFPs) were recorded in the PFC of WT, KO-1A, and KO-2A mice. PCP (10 mg/kg, intraperitoneally) reduced SCO equally in WT, KO-2A, and KO-1A mice (58±4%, 42±7%, and 63±7% of pre-drug values, n=23, 13, 11, respectively; p<0.0003). Clozapine (0.5 mg/kg, intraperitoneally) significantly reversed PCP effect in WT and KO-2A mice, but not in KO-1A mice nor in WT mice pretreated with the selective 5-HT1AR antagonist WAY-100635.The PCP-induced disorganization of PFC activity does not appear to depend on serotonergic function. However, the lack of effect of clozapine in KO-1A mice and the prevention by WAY-100635 indicates that its therapeutic action involves 5-HT1AR activation without the need to block 5-HT2AR, as observed with clozapine-induced cortical dopamine release.
Similar content being viewed by others
INTRODUCTION
The prefrontal cortex (PFC) plays a fundamental role in higher brain functions (Fuster, 1997; Miller and Cohen, 2001). Numerous observations suggest an abnormal function of this cortical area in schizophrenia (Elvevag and Goldberg, 2000; Goldman-Rakic, 1994; Lewis and Anderson, 1995; Uhlhaas and Singer, 2010; Uhlhaas and Singer, 2006; Winterer et al, 2004). In particular, anatomical, cellular, and neurochemical alterations have been reported in the frontal lobe of patients with schizophrenia (Harrison, 1999; Lewis and Lieberman, 2000; Lewis et al, 2005; Selemon and Goldman-Rakic, 1999).
Non-competitive NMDA receptor (NMDA-R) antagonists such as phencyclidine (PCP) or ketamine exacerbate clinical symptoms in patients with schizophrenia and induce behavioral alterations that resemble schizophrenia symptoms in healthy individuals and experimental animals (Handelmann et al, 1987; Hudzik and Wenger, 1993; Jentsch and Roth, 1999; Krystal et al, 1994; Krystal et al, 2003; Pradhan, 1984; Stefani and Moghaddam, 2005; Verma and Moghaddam, 1996). In particular, acute PCP administration to humans induces a schizophrenia-like state (Allen and Young, 1978; Bakker and Amini, 1961; Castellani et al, 1982; Javitt and Zukin, 1991; Luby et al, 1959; Steinpreis, 1996). Overall, these observations have led to the use of these agents as pharmacological models of schizophrenia (Javitt and Zukin, 1991; Krystal et al, 1994; Malhotra et al, 1997; Newcomer et al, 1999). Clozapine (CLZ) the prototype of atypical antipsychotic drugs attenuates the behavioral and cognitive impairments induced by the acute administration of non-competitive NMDA-R antagonists (Geyer et al, 2001; Idris et al, 2005; Lipina et al, 2005).
Previous results indicate that acute PCP administration induces a profound loss of cortical synchrony at low frequencies (slow cortical oscillations, SCO; 0.15–4 Hz), an effect reversed by the subsequent administration of classical (haloperidol) and atypical (CLZ) antipsychotic drugs (Kargieman et al, 2007). PCP also exerts similar alterations in thalamic nuclei interconnected with the PFC, which are also reversed by CLZ administration (Santana et al, 2011). Interestingly, the hallucinogen DOI, a preferential 5-HT2A receptor (5-HT2AR) agonist, induced a comparable loss of SCO in PFC, an effect also reversed by haloperidol and CLZ (Celada et al, 2008). Overall, these observations suggest that the reduction in SCO and its reversal by antipsychotic drugs may be a useful model to identify new targets in antipsychotic drug development.
5-HT1A receptor (5-HT1AR) and 5-HT2AR are abundantly expressed in rodent PFC (Pompeiano et al, 1992, 1994; Santana et al, 2004), where they are mostly co-expressed (Amargos-Bosch et al, 2004). They mediate opposing actions (inhibitions—5-HT1AR; excitations—5-HT2AR) of 5-HT and selective agonists (Amargos-Bosch et al, 2004; Araneda and Andrade, 1991; Marek and Aghajanian, 1999; Puig et al, 2005). CLZ shows an affinity for 5-HT2AR greater than that for dopamine D2 receptors (Meltzer, 1999). Likewise, despite showing moderate in vitro affinity for 5-HT1AR, CLZ and other atypical antipsychotic drugs behave as agonists at 5-HT1AR in vivo to increase PFC dopamine release (Bortolozzi et al, 2010; Diaz-Mataix et al, 2005; Ichikawa et al, 2001; Rollema et al, 1997).
In this study, we examined whether PCP also alters SCO in mouse PFC. Moreover, taking advantage of the availability of mice lacking 5-HT1AR and 5-HT2AR, we further examined the involvement of such serotonin (5-HT) receptors in the mechanism of action of PCP and CLZ in the above-mentioned effects on SCO.
MATERIALS AND METHODS
Animals and Treatments
C57BL/6 (wild-type, WT) mice were used. Male homozygous 5-HT1AR knockout (hereafter referred as KO-1A) mice and male homozygous 5-HT2A receptor knockout mice (hereafter referred as KO-2A) were also used. Both strains were on a C57/BL6 background. Generation of both KO strains has been reported elsewhere (Fiorica-Howells et al, 2002; Parks et al, 1998; Weisstaub et al, 2006). From these initial sources, mice were transferred to the animal facility of the University of Barcelona School of Medicine, where stable colonies were grown. Animals were kept in a controlled environment (12 h light–dark cycle and 22±2 °C room temperature) with food and water provided ad libitum. Animal care followed the European Union regulations (OJ of EC L358/1 18/12/1986) and was approved by the Institutional Animal Care and Use Committee.
PCP and CLZ were from Sigma/RBI (Natick, MA). Doses are expressed as free bases. Mice were injected intraperitoneally with the following treatments: (a) PCP (10 mg/kg), followed by CLZ (0.5 mg/kg); (b) PCP (10 mg/kg), followed by saline; and (c) saline, followed by CLZ (0.5 mg/kg). Time between injections was 10–12 min. The dose of CLZ was chosen from the literature owing to its ability to antagonize behavioral effects of NMDA-R antagonists (Bradford et al, 2010; Gleason and Shannon, 1997; Mutlu et al, 2011; Scorza et al, 2010; Yadav et al, 2011).
To further evaluate the role of the 5-HT1AR on CLZ-induced reversal of PCP actions on PFC, we performed an additional experiment in which mice were pretreated with saline or the selective 5-HT1AR antagonist WAY-100635 (0.3 mg/kg, subcutaneously) 30 min before CLZ administration, as used previously (Duvvuri et al, 2009).
At the end of the experiments, mice were killed by anesthetic overdose and the placement of recording electrodes verified histologically.
Local Field Potential Recordings
We examined drug effects on local field potential (LFP). LFP recordings provide a measure of population neuronal activity around the tip of the recording electrode (Schroeder and Lakatos, 2009; Shoham and Nagarajan, 2003) and allow to assess oscillatory electrical activity.
Mice were anesthetized with chloral hydrate (400 mg/kg, intraperitoneally) and positioned in a David Kopf stereotaxic frame. Additional doses of chloral hydrate (40 mg/kg) were administered intraperitoneally. Body temperature was maintained at 37 °C with a heating pad. LFPs were recorded with glass micropipettes pulled from 2 mm capillary glass (World Precision Instruments, Sarasota, FL) on a Narishige (Tokyo, Japan) PE-2 pipette puller. The electrode impedance was between 4 and 8 MΩ. The signal was amplified ( × 10) with a Neurodata IR283 (Cygnus Technology, Delaware Water Gap, PA), post-amplified ( × 100) and filtered using a band-pass filter (0.1–100 Hz) (Cibertec amplifier/filter, Madrid, Spain), and computed online using a DAT 1401plus interface system Spike2 software (CED). Descents in the medial PFC (mPFC) were carried out at AP +2.2 to +2.4, L −0.2 to −0.4, and DV −1.0 to −2.5 below brain surface. Stereotaxic coordinates were taken from bregma and duramater according to the mouse brain atlas (Franklin and Paxinos, 1997). After recording stable baseline activity for 5 min, PCP (10 mg/kg in saline) was slowly (15 s) administered intraperitoneally. CLZ (0.5 mg/kg, intraperitoneally) was injected 10 min after PCP administration. At the end of experiments, mice were killed by an overdose of anesthetic.
Data Analysis
The effect of PCP was examined in WT (n=23), KO-1A (n=11), and KO-2A (n=13) mice. Off-line analysis was performed using the SPIKE 2 software (Cambridge Electronic Design, Cambridge, UK). Power spectra were constructed by using fast Fourier transformation of 1-min signal intervals (band-pass filter of 0.1–100 Hz) corresponding to baseline, PCP, and PCP+CLZ. Power resolution was 0.15 Hz. Analyses of drug effects on SCO (0.15–4 Hz) and on the dominant wave (∼1 Hz) of SCO were measured separately. Results are given as AUCs.
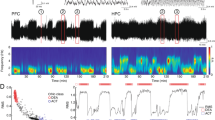
In addition, we measured the number of active phases in LFP recordings using a script of the Spike2 software. As observed in Figure 1a in basal conditions, the discharge of PFC pyramidal neurons occurs only during the active phase of the LFP, corresponding to depolarized or ‘UP’ states recorded intracellularly (Steriade et al, 1993a,1993b). Each downward deflection in the LFP trace was considered an active (or UP) phase when the time between two adjacent events was >250 ms (eg, <4 Hz; upper limit of SCO), and the amplitude of the deflection was three times the amplitude of the inactive phase. The actual recordings, as well as the number of active (UP) phases, transcribed as events, are shown in Figure 1a.
(a) Lower trace and mid-trace show action potential recorded extracellularly and local field potential (LFP) in mouse prefrontal cortex (PFC) in basal conditions. The upper trace depicts the number of UP states (see Materials and methods). Time bar is 5 s. (b) Power spectrum of the example is shown in (a). Note the predominance of the oscillation close to 1 Hz. (c–e) Correlations found between the individual basal values of three variables used in this study, (c) shows the correlation between the area under the curves (AUCs) of the power spectra of the 1 Hz oscillation (abscissa) with that of slow cortical oscillations (SCO) (0.15–4 Hz) (ordinate) (r=0.99; n=31). (d and e) show, respectively, the correlations between the AUCs of SCO and of the 1 Hz oscillation (abscissa) with the number of UP states (ordinate) (r=0.82 and 0.81, respectively; n=31).
The power spectra and the number of active phases were analyzed in 1-min time periods corresponding to baseline conditions (immediately before PCP administration), after PCP (10 min post-administration), and PCP+CLZ (10 min after CLZ administration). These time windows were selected on the basis of the behavioral effects of intraperitoneal administration of PCP, which start 3–4 min after injection and last for more than 20 min, as observed in non-anesthetized mice. Drug effects were analyzed using ANOVA, followed by post-hoc t-tests (Newman–Keuls) or paired Student's t-test, as appropriate. Statistical significance was set at the 95% confidence level (two-tailed). Data are given as means±SEM.
RESULTS
Characteristics of SCO in Mouse mPFC
LFP recordings of mouse mPFC in baseline conditions show the presence of characteristic SCO (0.15–4 Hz) with a marked predominance of a sharp wave at ∼1 Hz (Figure 1). As observed in other species (Kargieman et al, 2007; Mukovski et al, 2007; Steriade et al, 1993b), action potentials were discharged in temporal coincidence with active phases of LFP (Figure 1a). Individual differences in SCO and in the ∼1 Hz oscillations were paralleled by changes in the number of active phases. Significant correlations were found between the basal values of these variables: (i) SCO (0.15–4 Hz) vs 1 Hz oscillation (r=0.99; p<0.0001); (ii) SCO (0.15–4 Hz) vs number of active (UP) phases (r=0.82; p<0.0001); and (iii) 1 Hz oscillation vs number of active (UP) phases (r=0.81; p<0.0001) (n=31; Figure 1). The high correlation between the individual values of SCO and the 1 Hz oscillation indicates that the latter wave is the main contribution to SCO in mice PFC.
The power spectra of SCO did not differ between genotypes (WT: 0.024±0.003; KO-1A: 0.016±0.003; and KO-2A: 0.017±0.004 μV2; (F(2, 44)=1.67, p=0.20), n=23, 11, and 13, respectively). Likewise, the power spectra of the 1 Hz oscillation (WT: 0.018±0.002; KO-1A: 0.011±0.002; and KO-2A: 0.012±0.003 μV2; n=23, 11, and 13, respectively) and the number of active phases per min (WT: 25.83±1.81; KO-1A: 22.9±3.31; and KO-2A: 18.54±2.68; n=23, 11, and 13, respectively) did not significantly differ among genotypes (F(2, 44)=2.55, p=0.09) and (F(2, 44)=2.44, p=0.1) for 1 Hz oscillation and the number of active phases per minute, respectively. The three variables (power spectra of SCO and ∼1 Hz wave as well as number of active phases) were equally sensitive to drug effects (see below).
Effect of PCP on mPFC Oscillations
As previously reported in rats (Kargieman et al, 2007), the systemic administration of PCP to WT mice reduced SCO, from 0.024±0.003 to 0.013±0.002 μV2 (58.4±4.1% of baseline; p<0.00001; paired Student's t-test, n=23). This reduction was paralleled by a similar decrease in the number of actives phases (from 25.8±1.8 to 14.1±1.1; 54.6% of baseline; p<0.000001, Student's t-test, n=23). Figure 2 shows a representative example of the effect of PCP on SCO as well as the average effects on power spectra and number of active phases.
Effect of phencyclidine (PCP) administration on slow cortical oscillations (SCO). (a) Local field potential (LFP) recording and power spectra of a representative experiment showing the decrease in SCO after PCP administration. Small bars below the LFP recording denote the one 1-min period corresponding to the power spectrums shown below. Note that PCP effect persisted for at least 25 min. (b) Spectrograms showing the effects of the administration of PCP and saline in the time periods shown in (a). Time bar in abscissa are 10 s; ordinates are in Hz. The intensity of the power spectrum is color-coded (red=high intensity; blue=low intensity). (c) Bar graph showing the effects of PCP on SCO, and on the number of active phases per minute. *p<0.00001 vs baseline; n=23.
To examine the kinetics of PCP effect on SCO in mice, we measured the effect of PCP at 10 and 20 min post-administration in six experiments. Changes in PFC activity started at approximately 4 min after intraperitoneal administration of PCP and persisted for at least 20 min (Figures 2 and 3). One-way repeated-measures ANOVA of the power spectra of SCO revealed a significant effect of PCP at 10 and 20 min after administration (0.025±0.007, 0.017±0.006, and 0.012±0.004 μV2 in basal conditions and 10 and 20 min after PCP, respectively ((F(2, 10)=9.47, p<0.005), n=6), with no significant differences between 10 and 20 min (Figures 2a and 3). Similarly, there were no differences between the number of active phases at 10 and 20 min (22.8±3.3, 13.5±3.3, and 12.7±3.3) events per min in basal conditions and after 10 and 20 min of PCP administration, respectively ((F(2, 10)=18.56, p<0.0004), n=6; Figure 3).
Bar graphs showing the effects of phencyclidine (PCP) on slow cortical oscillations (SCO) (a), on the ∼1 Hz oscillation (b), and on the number of active phases per minute (c) 10 and 20 min after administration. *p<0.05, **p<0.005 vs basal.
Saline injections did not alter SCO, 1 Hz oscillations, nor the number of active phases (data not shown). Also, saline injections did not modify the SCO suppression induced by the subsequent PCP administration (Figure 2).
Effect of PCP on mPFC Oscillations in KO-1A and KO-2A Mice
Systemic PCP administration reduced SCO (0.15–4 Hz) similarly in the mPFC of WT, KO-1A, and KO-2A mice (Figures 2 and 4). Two-way ANOVA revealed a significant effect of PCP ((F(1, 44)=50.00, p<0.0001), n=23, 11, and 13 for WT, KO-1A, and KO-2A mice, respectively), with no significant effects of genotype and treatment × genotype interaction.
Effect of phencyclidine (PCP) on slow cortical oscillations (SCO) in the prefrontal cortex (PFC) of 5-HT2A receptor (5-HT2AR)- and 5-HT1AR-knockout (KO) mice (KO-2A and KO-1A, respectively). Representative effect of PCP on SCO in the PFC of a KO-2A mouse (a) and a KO-1A mouse (c). Power spectrum show a decrease of SCO (0.15–4 Hz) after intraperitoneal (i.p.) PCP administration. Bar graph shows the effect of PCP on SCO and on the number of active phases per minute in KO-2A mice (b) and KO-1A mice (d). *p<0.002; n=13 and 11, respectively.
The analyses of the effect of PCP on the 1 Hz oscillation also revealed a reduction of the power spectra in all genotypes. Two-way ANOVA revealed a significant effect of PCP ((F(1, 44)=38.87, p<0.0001), n=23, 11, and 13 for WT, KO-1A, and KO-2A mice, respectively), with no significant effects of genotype and treatment × genotype interaction.
As in WT mice, PCP administration also reduced the number of active phases in the mPFC of KO-1A and KO-2A mice, from 22.9±3.3 to 13.2±2.8 events per min (KO-1A, p<0.001; Student's t-test, n=11) and from 18.54±2.68 to 7.85±1.13 events per min (KO-2A, p<0.001; Student's t-test, n=13). Two-way ANOVA revealed a significant effect of PCP ((F(1, 44)=126.05, p<0.0001), n=23, 13, and 11 for WT, KO-2A and KO-1A mice, respectively) and genotype (F(2, 44)=3.51, p<0.05), but no significant effect of treatment × genotype interaction (Figure 4).
CLZ Reversal of PCP Effect
Next, we next examined whether (i) CLZ could reverse PCP effects on SCO in the PFC of WT mice, as observed in rats (Kargieman et al, 2007), and (ii) whether CLZ effects were comparable in all genotypes (WT, KO-1A, and KO-2A).
WT Mice
As observed in rats (Kargieman et al, 2008), CLZ (0.5 mg/kg, intraperitoneally) had no effect by itself on SCO in WT mice (113% of basal values; NS, n=4).
In the WT mice treated with PCP+CLZ (n=19), PCP reduced SCO to 59.9±4.4% of baseline, an effect significantly reversed by CLZ administration (Figure 5). One-way repeated-measures ANOVA revealed a significant effect of treatment (F(2, 36)=30.82; p<0.00001), with significant post-hoc differences between PCP vs baseline and PCP+CLZ vs PCP, indicating that CLZ reversed PCP effects on SCO in mouse PFC.
Reversal by clozapine (CLZ) of the phencyclidine (PCP)-induced reduction in slow cortical oscillations (SCO), ∼1 Hz cortical oscillations, and number of active per minute. *p<0.0002 vs basal, #p<0.01 vs PCP, and ##p<0.0005 vs PCP.
When the effects of PCP and CLZ were assessed by the change of the ∼1 Hz wave or the number of active phases, the results were similar. Thus, PCP reduced the ∼1 Hz wave to 55.4±4.7% of baseline, an effect reversed by CLZ ((F(2, 36)=35.22; p<0.00001), n=19; one-way repeated-measures ANOVA), with significant post-hoc differences between PCP vs baseline and PCP+CLZ vs PCP (Figure 5). Likewise, CLZ reversed the PCP-evoked reduction in the number of active phases ((F(2, 36)=22.11; p<0.00001), n=19; one-way ANOVA), with significant post-hoc differences between PCP vs baseline and PCP+CLZ vs PCP (Figure 5).
Genotype Comparisons
As observed in WT mice, the administration of CLZ (0.5 mg/kg, intraperitoneally) significantly reversed PCP effects on SCO in KO-2A mice, but failed to do it in KO-1A mice (Figure 6). Two-way ANOVA (treatment, genotype) analysis of the SCO data in all genotypes revealed a significant effect of treatment (F(2, 78)=39.9, p<0.0001) and significant treatment × genotype interaction ((F(4, 78)=13.09, p<0.00001), n=19, 11, and 12 for WT, KO-1A, and KO-2A mice, respectively). Post-hoc comparisons showed significant differences between PCP vs baseline activity in all genotypes and significant post-hoc differences between PCP+CLZ treatment vs treatment with PCP alone in WT mice and in KO-2A mice, but not in KO-1A mice (Figure 6).
Reversal by clozapine of phencyclidine (PCP) effects on cortical oscillation in the three genotypes. (a) Graphs showing the average effects of PCP and PCP+clozapine (CLZ) as assessed by the area under the curves (AUCs) of power spectra (upper graph) and the number of active phases (lower graph). Note the lack of CLZ reversal in knockout (KO)-1A mice. n=23, 11, and 12 for wild-type (WT), KO-1A, and KO-2A mice. *p<0.001 vs basal; #p<0.05 vs PCP; ##p<0.0005 vs PCP; αp<0.0002, α‘p<0.01 post-hoc test after two-way analysis of variance (ANOVA) (see text). (b) Representative examples of the power spectra of slow cortical oscillations (SCO) in mice prefrontal cortex (PFC) obtained in basal conditions, after PCP and after the subsequent CLZ administration (10 and 20 min after PCP administration, respectively) in the three genotypes. Note the marked reduction of the power of SCO in the three genotypes by PCP and the reversal induced by CLZ in WT and KO-2A mice, but not in KO-1A mice.
Likewise, on the number of active phases, two-way ANOVA revealed a significant effect of treatment (F(2, 78)=71.07, p<0.0001), genotype (F(2, 39)=9.13 p<0.001), and treatment × genotype interaction ((F(4, 78)=15.29; p<0.00001), n=19, 11, and 12 for WT, KO-1A, and KO-2A mice, respectively), with significant post-hoc differences between PCP vs baseline in all genotypes and significant post-hoc differences between PCP+CLZ vs PCP in WT mice and in KO-2A mice. On the contrary, post-hoc comparisons showed that CLZ administration did not significantly reverse the effect of PCP in KO-1A mice (Figure 6). So, CLZ significantly overturned PCP effect in WT and KO-2A mice, but not in KO-1A mice.
Prevention of CLZ Effect in WT Mice by the 5-HT1AR Antagonist WAY-100635
Pretreatment with the 5-HT1AR antagonist WAY-100635 (0.3 mg/kg, subcutaneously) prevented the CLZ reversal of PCP-evoked reduction on SCO without having any effect on SCO by itself. Two-way ANOVA revealed a significant effect of treatment (F(3, 30)=23.76, p<0.001) and of the treatment × pretreatment interaction ((F(3, 30)=3.88, p<0.02), n=6 for saline and WAY-100635 pretreatment, respectively), with significant post-hoc differences between PCP vs baseline in both groups. Likewise, Newman–Keuls post-hoc test revealed a significant difference in the effect of CLZ in saline and WAY-100635-pretreated mice (Figure 7).
The 5-HT1A receptor (5-HT1AR) antagonist WAY-100635 prevents the clozapine (CLZ) reversal of phencyclidine (PCP) effect on slow cortical oscillations (SCO) in wild-type (WT) mice. (a) Representative examples of local field potential (LFP) recording in prefrontal cortex (PFC) from mice pretreated with saline (a1) and WAY-100635 (0.3 mg/kg, subcutaneously) (a2). Spectrograms showing the effects of the administration of WAY-100635 or saline, PCP, and CLZ in the time periods shown in the upper scheme. The intensity of the power spectrum is color-coded (red=high intensity; blue=low intensity) (b and c) Bar graph showing the prevention by WAY-100635 (0.3 mg/kg, subcutaneously) pretreatment of CLZ reversal of PCP-evoked reduction on SCO (b) and on the number of active phases per minute (c). WAY-100635 by itself has not effect on both parameters. *p<0.02 vs basal, #p<0.01 saline-PCP-CLZ vs WAY-100635-PCP-CLZ.
Likewise, two-way ANOVA also revealed a significant effect of treatment (F(3, 30)=8.54, p<0.0003) and treatment × pretreatment interaction (F(3, 30)=5.02; p<0.01) (n=6 each group) on the number of active phases. As observed with power spectra, Newman–Keuls post-hoc test revealed a significant difference in the effect of CLZ in saline and WAY-100635-pretreated mice (Figure 6).
DISCUSSION
This study shows that PCP is able to alter SCO in mouse PFC, thus extending previous observations in rat PFC, indicating a general action of PCP on rodent brain. This allows the use of transgenic mice to examine the involvement of neurotransmitter receptors in drug effects. In this regard, an important and novel observation of this study is the requirement of 5-HT1AR activation for the CLZ-mediated reversal of PCP effects on SCO, indicating that 5-HT1AR play an important role in the therapeutic action of CLZ. Likewise, a new methodological contribution of this study is the use of the number of active (UP) phases as a new variable to examine drug effects on cortical synchrony.
These findings are consistent with previous reports of the effects of psychotomimetic agents (eg, PCP and DOI) on SCO in rat PFC (Celada et al, 2008; Kargieman et al, 2007) and thalamic nuclei projecting to PFC (Santana et al, 2011). Hence, PCP markedly alters SCO in the PFC of both rodent species (Kargieman et al, 2007; this study) and these effects are restored by classical (haloperidol) and atypical (CLZ) antipsychotic drugs (Kargieman et al, 2007).
Cortical neurons exhibit synchronous, hyperpolarized (resting or DOWN state), and plateau depolarized (UP state) membrane potential fluctuations during slow-wave sleep and in anesthetized animals (Steriade et al, 1993a,1993b; Lewis and O’Donnell, 2000; Mukovski et al, 2007). These changes can be observed at the population level as SCO in LFP and EEG recordings (Destexhe et al, 2007; Steriade et al, 1993b). The ∼1-Hz cortical oscillation, predominant in SCO, appears to be essential for information processing in PFC (Engel et al, 2001; Engel and Singer, 2001). Hence, boosting low EEG frequencies during sleep has been shown to improve memory (Marshall et al, 2006), and the disruption of cortical network synchrony may have a negative impact on working memory and in behavioral representation, two important PFC-dependent tasks altered in schizophrenia (Elvevag and Goldberg, 2000).
The study of cortical oscillatory activity has a high translational value for the study of the pathophysiology and treatment of schizophrenia, as well as in the identification of neurobiological targets for drug development (Ford et al, 2007). Although most neurophysiological studies assessing oscillatory activity in schizophrenic patients have focused on high-frequency oscillations (β, γ) (Kwon et al, 1999; Spencer et al, 2003; Uhlhaas and Singer, 2006), alterations in lower frequencies have also been reported (Basar-Eroglu et al, 2009; Shin et al, 2010), which may be relevant to the emergence of schizophrenia symptoms given the involvement of slow oscillations in cognitive processes and memory consolidation (Stickgold, 2005; Marshall et al, 2006).
Here we report that PCP administration markedly reduced synchrony in mouse PFC, as assessed by SCO (0.15–4 Hz), 1-Hz oscillations, and the number of active phases. These variables are correlated since they all reflect the synchronized alternating activity states of neuronal populations in the recording area and were equally sensitive to drug effects.
In normal conditions, action potentials are discharged synchronically in temporal coincidence with active (UP) phases (Kargieman et al, 2007; Mukovski et al, 2007; Steriade et al, 1993b; Figure 1), yet in the presence of PCP, cortical synchrony is markedly reduced or lost, whereas discharge rate is enhanced, leading to a profound temporal disorganization of PFC neuronal activity (Kargieman et al, 2007; this study).
The reduction of SCO and number of active phases is possibly due to an imbalance between excitatory and inhibitory neurotransmission within the PFC. In the rat PFC, PCP markedly increased (∼3-fold) the discharge rate of a 45% of pyramidal neurons and reduced it in another 33% (Kargieman et al, 2007). UP states are sustained by a balance of excitatory and inhibitory network interactions—including afferent modulatory inputs—and by intrinsic cellular mechanisms (Contreras and Steriade, 1995; Lewis and O’Donnell, 2000; O’Donnell and Grace, 1995; Sanchez-Vives and McCormick, 2000; Shu et al, 2003; Timofeev et al, 2000a, 2000b). Hence, the decrease in GABAergic activity reported in the NMDA-R model of schizophrenia (Gonzalez-Burgos and Lewis, 2008; Homayoun and Moghaddam, 2007; Krystal et al, 2003; Lewis et al, 2005) may account for this effect, assuming a preferential blockade of NMDA-R in GABAergic interneurons by PCP. Thus, the blockade of GABAA-mediated inhibitions with picrotoxin decreased the number of UP states (Cossart et al, 2003) and suppressed slow-wave oscillations (Shu et al, 2003). In support of this view, PCP mainly induced the expression of c-fos—a marker of neuronal activity—in cortical pyramidal neurons and on excitatory thalamic neurons, but not in GABA interneurons in PFC and subcortical areas reciprocally connected with PFC (Kargieman et al, 2007; Santana et al, 2011).
Overall, the present and past observations (Kargieman et al, 2007; Santana et al, 2011) indicate that PCP evokes a highly disorganized state in PFC, with an increased pyramidal discharge occurring in a random manner, without the temporal frame of the ∼1 Hz oscillation, an effect occurring also in thalamic neurons reciprocally connected with PFC (Santana et al, 2011). This pattern of action is similar to that observed in recent human neuroimaging studies with ketamine (Vollenweider and Kometer, 2010). Given the top–down control exerted by PFC on most cortical and subcortical areas (Miller and Cohen, 2001), the disruption of PFC and thalamic function by PCP may have a profound impact on a large number of brain functions, which may account for the perceptual, cognitive, affective, and motor actions of PCP (Krystal et al, 1994, 2003).
PCP increases 5-HT release in PFC (Amargos-Bosch et al, 2006) and endogenous 5-HT can excite or inhibit pyramidal cell activity via 5-HT2AR and 5-HT1AR, respectively (Araneda and Andrade, 1991; Puig et al, 2005). The similar effects of PCP in all genotypes indicate that changes in SCO occur upstream of the change in 5-HT release and without a significant involvement of such receptor populations.
In contrast, the effect of CLZ markedly differs among mouse genotypes. As in rat PFC, CLZ reversed PCP effect on SCO in WT and KO-2A mice. However, CLZ was without effect in KO-1A mice. Previous studies have demonstrated varying in vitro affinities of CLZ for 5-HT1AR, for which it behaves as a partial agonist. Hence, CLZ displays a modest affinity for 5-HT1AR (∼100 nM) (Kroeze et al, 2003; Roth et al, 2004), whereas affinities in the range 700–800 nM were reported previously (Arnt and Skarsfeldt, 1998; Bymaster et al, 1996). Despite this moderate–low in vitro affinity, CLZ exerts a number of its in vivo pharmacological actions—particularly the increase in PFC DA release—via 5-HT1AR activation (Bortolozzi et al, 2010; Diaz-Mataix et al, 2005; Ichikawa et al, 2001; Rollema et al, 1997), an observation that may have several interpretations.
5-HT1AR and 5-HT2AR are highly co-expressed in rat and mouse PFC neurons (Amargos-Bosch et al, 2004). Thus, although there are no compensatory changes of other 5-HT receptors in KO-1A and KO-2A mice (Amargos-Bosch et al, 2004; Bortolozzi et al, 2010; Popa et al, 2005), the blockade of 5-HT2AR by CLZ might alter the physiological balance between 5-HT1AR and 5-HT2AR, resulting in an increase of 5-HT1AR-mediated neurotransmission. However, these results, obtained in anesthetized mice, together with a recent report on the effect of CLZ on dopamine release in the PFC of awake mice (Bortolozzi et al, 2010) do not support this view, as CLZ reversed the effects of PCP on SCO in KO-2A mice, whereas it did not in KO-1A mice. Moreover, the observation that WAY-100635 pretreatment prevented the CLZ-induced reversal of PCP effects on SCO suggests a direct interaction of CLZ (or its main metabolite N-desmethylclozapine) with 5-HT1AR. This view agrees with PET scan data, indicating the partial displacement of [11C]WAY-100635 (a hardly displaceable ligand) by CLZ in monkey brain (Chou et al, 2003). This apparent discrepancy between in vitro and in vivo data may be also accounted for by the contribution of the main CLZ metabolite, N-desmethylclozapine, which shows 5-HT1AR affinity in the 10 nM range (http://www.kidb.case.edu/pdsp.php).
The requirement of 5-HT1AR activation by CLZ to restore SCO is entirely similar to its ability to enhance PFC dopamine release, an effect thought to be involved in the superior efficacy of CLZ on negative symptoms and cognitive deficits (Leucht et al, 2009a, 2009b). Hence, CLZ and other atypical antipsychotic drugs (but not classical dopamine D2 blockers) share the ability to increase DA release in rodent mPFC through 5-HT1AR activation (Bortolozzi et al, 2007; Diaz-Mataix et al, 2005; Ichikawa et al, 2001; Li et al, 2009; Rollema et al, 1997, 2000), an effect depending on the exclusive activation of 5-HT1AR in mPFC as observed in KO mice (Bortolozzi et al, 2007, 2010; Diaz-Mataix et al, 2005). These results also agree with a recent report indicating that CLZ's ability to normalize NMDA-R hypofunction in vivo depends on an intact presynaptic serotonergic function and that 5-HT2AR are not essential for CLZ reversal of PCP-induced disruption of sensory–motor gating (Yadav et al, 2011).
Irrespectively of the mechanisms involved, the requirement of 5-HT1AR for CLZ to increase dopamine release in PFC and to restore SCO suggests an association between both effects that requires further investigation. Hence, the dopamine increase may restore the loss of SCO via activation of D1 receptors in fast-spiking interneurons (Tseng et al, 2006). In support of the involvement of GABA interneurons in the effect of CLZ, the combination of PCP and CLZ induced the expression of c-fos in PFC GABAergic neurons (Kargieman et al, 2007; Santana et al, 2011), suggesting that CLZ may restore cortical synchrony via an enhancement of GABA inputs onto pyramidal neurons excited by PCP.
In summary, this study shows for the first time that PCP markedly reduces cortical synchrony in mouse PFC. The experiments carried out in KO mice illustrate the validity of this model to examine the involvement of neurotransmitter receptors in drug effects and indicate that the actions of CLZ to restore cortical synchrony require the presence of 5-HT1AR, as observed using other experimental models, such as the cortical release of dopamine (Bortolozzi et al, 2010). Overall, this adds further support to the involvement of this 5-HT receptor in antipsychotic drug action (Newman-Tancredi, 2010).
References
Allen RM, Young SJ (1978). Phencyclidine-induced psychosis. Am J Psychiatry 135: 1081–1084.
Amargos-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P et al (2004). Co-expression and in vivo interaction of serotonin(1A) and serotonin(2A) receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex 14: 281–299.
Amargos-Bosch M, Lopez-Gil X, Artigas F, Adell A (2006). Clozapine and olanzapine, but not haloperidol, suppress serotonin efflux in the medial prefrontal cortex elicited by phencyclidine and ketamine. Int J Neuropsychopharmcol 9: 565–573.
Araneda R, Andrade R (1991). 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 40: 399–412.
Arnt J, Skarsfeldt T (1998). Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology 18: 63–101.
Bakker C, Amini F (1961). Observations on the psychotomimetic effects of sernyl. Compr Psychiatry 2: 269–280.
Basar-Eroglu C, Schmiedt-Fehr C, Mathes B, Zimmermann J, Brand A (2009). Are oscillatory brain responses generally reduced in schizophrenia during long sustained attentional processing? Int J Psychophysiol 71: 75–83.
Bortolozzi A, Diaz-Mataix L, Toth M, Celada P, Artigas F (2007). In vivo actions of aripiprazole on serotonergic and dopaminergic systems in rodent brain. Psychopharmacology (Berl) 191: 745–758.
Bortolozzi A, Masana M, Díaz-Mataix L, Cortés R, Scorza MC, Gingrich JA et al (2010). Dopamine release induced by atypical antipsychotics in prefrontal cortex requires 5-HT(1A) receptors but not 5-HT(2A) receptors. Int J Neuropsychopharmacol 13: 1299–1314.
Bradford AM, Savage KM, Jones DN, Kalinichev M (2010). Validation and pharmacological characterisation of MK-801-induced locomotor hyperactivity in BALB/C mice as an assay for detection of novel antipsychotics. Psychopharmacology (Berl) 212: 155–170.
Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC et al (1996). Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 14: 87–96.
Castellani S, Giannini AJ, Boeringa JA, Adams PM (1982). Phencyclidine intoxication: assessment of possible antidotes. J Toxicol Clin Toxicol 19: 313–319.
Celada P, Puig MV, Diaz-Mataix L, Artigas F (2008). The hallucinogen DOI reduces low-frequency oscillations in rat prefrontal cortex: reversal by antipsychotic drugs. Biol Psychiatry 64: 392–400.
Chou YH, Halldin C, Farde L (2003). Occupancy of 5-HT1A receptors by clozapine in the primate brain: a PET study. Psychopharmacology (Berl) 166: 234–240.
Contreras D, Steriade M (1995). Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci 15: 604–622.
Cossart R, Aronov D, Yuste R (2003). Attractor dynamics of network UP states in the neocortex. Nature 423: 283–288.
Destexhe A, Hughes SW, Rudolph M, Crunelli V (2007). Are corticothalamic ‘up’ states fragments of wakefulness? Trends Neurosci 30: 334–342.
Diaz-Mataix L, Scorza MC, Bortolozzi A, Toth M, Celada P, Artigas F (2005). Involvement of 5-HT1A receptors in prefrontal cortex in the modulation of dopaminergic activity: role in atypical antipsychotic action. J Neurosci 25: 10831–10843.
Duvvuri V, Risbrough VB, Kaye WH, Geyer MA (2009). 5-HT1A receptor activation is necessary for 5-MeODMT-dependent potentiation of feeding inhibition. Pharmacol Biochem Behav 93: 349–353.
Elvevag B, Goldberg TE (2000). Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol 14: 1–21.
Engel AK, Fries P, Singer W (2001). Dynamic predictions: oscillations and synchrony in top–down processing. Nat Rev Neurosci 2: 704–716.
Engel AK, Singer W (2001). Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci 5: 16–25.
Fiorica-Howells E, Hen R, Gingrich J, Li Z, Gershon MD (2002). 5-HT(2A) receptors: location and functional analysis in intestines of wild-type and 5-HT(2A) knockout mice. Am J Physiol Gastrointest Liver Physiol 282: G877–G893.
Ford JM, Krystal JH, Mathalon DH (2007). Neural synchrony in schizophrenia: from networks to new treatments. Schizophr Bull 33: 848–852.
Franklin KBJ, Paxinos G (1997). The Mouse Brain in Stereotaxic. Academic Press: San Diego, CA.
Fuster JM (1997). The Prefrontal Cortex. Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. Lipincott-Raven: Philadelphia, New York.
Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR (2001). Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology 156: 117–154.
Gleason SD, Shannon HE (1997). Blockade of phencyclidine-induced hyperlocomotion by olanzapine, clozapine and serotonin receptor subtype selective antagonists in mice. Psychopharmacology (Berl) 129: 79–84.
Goldman-Rakic PS (1994). Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci 6: 348–357.
Gonzalez-Burgos G, Lewis DA (2008). GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull 34: 944–961.
Handelmann GE, Contreras PC, O’Donohue TL (1987). Selective memory impairment by phencyclidine in rats. Eur J Pharmacol 140: 69–73.
Harrison PJ (1999). The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain 122: 593–624.
Homayoun H, Moghaddam B (2007). NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27: 11496–11500.
Hudzik TJ, Wenger GR (1993). Effects of drugs of abuse and cholinergic agents on delayed matching-to-sample responding in the squirrel monkey. J Pharmacol Exp Ther 265: 120–127.
Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O’Laughlin IA, Meltzer HY (2001). 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem 76: 1521–1531.
Idris NF, Repeto P, Neill JC, Large CH (2005). Investigation of the effects of lamotrigine and clozapine in improving reversal-learning impairments induced by acute phencyclidine and D-amphetamine in the rat. Psychopharmacology (Berl) 179: 336–348.
Javitt DC, Zukin SR (1991). Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148: 1301–1308.
Jentsch JD, Roth RH (1999). The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 20: 201–225.
Kargieman L, Santana N, Mengod G, Celada P, Artigas F (2007). Antipsychotic drugs reverse the disruption in prefrontal cortex function produced by NMDA receptor blockade with phencyclidine. Proc Natl Acad Sci USA 104: 14843–14848.
Kargieman L, Santana N, Mengod G, Celada P, Artigas F (2008). NMDA antagonist and antipsychotic actions in cortico-subcortical circuits. Neurotoxicol Res 14: 129–140.
Kroeze WK et al (2003). H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology 28: 519–526.
Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R (2003). NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 169: 215–233.
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD et al (1994). Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans—psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51: 199–214.
Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG et al (1999). Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry 56: 1001–1005.
Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM (2009a). Second-generation vs first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 373: 31–41.
Leucht S, Komossa K, Rummel-Kluge C, Corves C, Hunger H, Schmid F et al (2009b). A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry 166: 152–163.
Lewis BL, O’Donnell P (2000). Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential ‘up’ states in pyramidal neurons via D(1) dopamine receptors. Cereb Cortex 10: 1168–1175.
Lewis DA, Anderson SA (1995). The functional architecture of the prefrontal cortex and schizophrenia. Psychol Med 25: 887–894.
Lewis DA, Hashimoto T, Volk DW (2005). Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6: 312–324.
Lewis DA, Lieberman JA (2000). Catching up on schizophrenia: natural history and neurobiology. Neuron 28: 325–334.
Li Z, Prus AJ, Dai J, Meltzer HY (2009). Differential effects of M1 and 5-hydroxytryptamine1A receptors on atypical antipsychotic drug-induced dopamine efflux in the medial prefrontal cortex. J Pharmacol Exp Ther 330: 948–955.
Lipina T, Labrie V, Weiner I, Roder J (2005). Modulators of the glycine site on NMDA receptors, D-serine and ALX 5407, display similar beneficial effects to clozapine in mouse models of schizophrenia. Psychopharmacology (Berl) 179: 54–67.
Luby ED, Cohen D, Rosenbaum G, Gottlieb J, Kelley R (1959). Study of a new schizophrenomimetic drug: sernyl. AMA Arch Neurol Psychiatry 81: 363–369.
Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D et al (1997). Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology 17: 141–150.
Marek GJ, Aghajanian GK (1999). 5-HT2A receptor or alpha1-adrenoceptor activation induces excitatory postsynaptic currents in layer V pyramidal cells of the medial prefrontal cortex. Eur J Pharmacol 367: 197–206.
Marshall L, Helgadottir H, Molle M, Born J (2006). Boosting slow oscillations during sleep potentiates memory. Nature 444: 610–613.
Meltzer HY (1999). The role of serotonin in antipsychotic drug action. Neuropsychopharmacology 21: 106S–115S.
Miller EK, Cohen JD (2001). An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202.
Mukovski M, Chauvette S, Timofeev I, Volgushev M (2007). Detection of active and silent states in neocortical neurons from the field potential signal during slow-wave sleep. Cereb Cortex 17: 400–414.
Mutlu O, Ulak G, Celikyurt IK, Akar FY, Erden F (2011). Effects of olanzapine, sertindole and clozapine on learning and memory in the Morris water maze test in naive and MK-801-treated mice. Pharmacol Biochem Behav 98: 398–404.
Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T et al (1999). Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology 20: 106–118.
Newman-Tancredi A (2010). The importance of 5-HT1A receptor agonism in antipsychotic drug action: rationale and perspectives. Curr Opin Invest Drugs 11: 802–812.
O’Donnell P, Grace AA (1995). Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci 15: 3622–3639.
Parks CL, Robinson PS, Sibille E, Shenk T, Toth M (1998). Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci USA 95: 10734–10739.
Pompeiano M, Palacios JM, Mengod G (1992). Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci 12: 440–453.
Pompeiano M, Palacios JM, Mengod G (1994). Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res 23: 163–178.
Popa D, Léna C, Fabre V, Prenat C, Gingrich J, Escourrou P, Hamon M et al (2005). Contribution of 5-HT2 receptor subtypes to sleep–wakefulness and respiratory control, and functional adaptations in knock-out mice lacking 5-HT2A receptors. J Neurosci 25: 11231–11238.
Pradhan SN (1984). Phencyclidine (PCP): some human studies. Neurosci Biobehav Rev 8: 493–501.
Puig MV, Artigas F, Celada P (2005). Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphe stimulation in vivo: involvement of serotonin and GABA. Cereb Cortex 15: 1–14.
Rollema H, Lu Y, Schmidt AW, Sprouse JS, Zorn SH (2000). 5-HT(1A) receptor activation contributes to ziprasidone-induced dopamine release in the rat prefrontal cortex. Biol Psychiatry 48: 229–237.
Rollema H, Lu Y, Schmidt AW, Zorn SH (1997). Clozapine increases dopamine release in prefrontal cortex by 5-HT1A receptor activation. Eur J Pharmacol 338: R3–R5.
Roth BL, Sheffler DJ, Kroeze WK (2004). Magic shotguns vs magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov 3: 353–359.
Sanchez-Vives MV, McCormick DA (2000). Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 3: 1027–1034.
Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F (2004). Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex 14: 1100–1109.
Santana N, Troyano-Rodriguez E, Mengod G, Celada P, Artigas F (2011). Activation of thalamocortical networks by the N-methyl-D-aspartate receptor antagonist phencyclidine: reversal by clozapine. Biol Psychiatry 69: 918–927.
Schroeder CE, Lakatos P (2009). Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci 32: 9–18.
Scorza MC, Castane A, Bortolozzi A, Artigas F (2010). Clozapine does not require 5-HT1A receptors to block the locomotor hyperactivity induced by MK-801 Clz and MK-801 in KO1A mice. Neuropharmacology 59: 112–120.
Selemon LD, Goldman-Rakic PS (1999). The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 45: 17–25.
Shin YW, Krishnan G, Hetrick WP, Brenner CA, Shekhar A, Malloy FW et al (2010). Increased temporal variability of auditory event-related potentials in schizophrenia and Schizotypal Personality Disorder. Schizophr Res 124: 110–118.
Shoham S, Nagarajan S (2003). The theory of central nervous system recording. In: Horch KW, Dhillon GS (eds). Neuroprosthetics: Theory and Practice. World Scientific Publishing: Singapore. pp 448–465.
Shu Y, Hasenstaub A, McCormick DA (2003). Turning on and off recurrent balanced cortical activity. Nature 423: 288–293.
Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW (2003). Abnormal neural synchrony in schizophrenia. J Neurosci 23: 7407–7411.
Stefani MR, Moghaddam B (2005). Transient N-methyl-D-aspartate receptor blockade in early development causes lasting cognitive deficits relevant to schizophrenia. Biol Psychiatry 57: 433–436.
Steinpreis RE (1996). The behavioral and neurochemical effects of phencyclidine in humans and animals: some implications for modeling psychosis. Behav Brain Res 74: 45–55.
Steriade M, McCormick DA, Sejnowski TJ (1993a). Thalamocortical oscillations in the sleeping and aroused brain. Science 262: 679–685.
Steriade M, Nunez A, Amzica F (1993b). A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci 13: 3252–3265.
Stickgold R (2005). Sleep-dependent memory consolidation. Nature 437: 1272–1278.
Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M (2000a). Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex 10: 1185–1199.
Timofeev I, Grenier F, Steriade M (2000b). Impact of intrinsic properties and synaptic factors on the activity of neocortical networks in vivo. J Physiol (Paris) 94: 343–355.
Tseng KY, Mallet N, Toreson KL, Le Moine C, Gonon F, O’Donnell P (2006). Excitatory response of prefrontal cortical fast-spiking interneurons to ventral tegmental area stimulation in vivo. Synapse 59: 412–417.
Uhlhaas PJ, Singer W (2006). Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron 52: 155–168.
Uhlhaas PJ, Singer W (2010). Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11: 100–113.
Verma A, Moghaddam B (1996). NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci 16: 373–379.
Vollenweider FX, Kometer M (2010). The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 11: 642–651.
Weisstaub NV, Zhou M, Lira A, Lambe E, González-Maeso J, Hornung JP et al (2006). Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 313: 536–540.
Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE et al (2004). Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am J Psychiatry 161: 490–500.
Yadav PN, Abbas AI, Farrell MS, Setola V, Sciaky N, Huang XP et al (2011). The presynaptic component of the serotonergic system is required for clozapine's efficacy. Neuropsychopharmacology 36: 638–651.
Acknowledgements
The work leading to these results has received funding from the Innovative Medicines Initiative Joint Undertaking (IMI) under Grant Agreement No. 115008 (NEWMEDS). IMI is a public–private partnership between the European Union and the European Federation of Pharmaceutical Industries and Associations. This work was supported by Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM) and Grants SAF 2007-62378, FIS PI09/1245 (PN de I+D+I 2008–2011, ISCIII-Subdirección General de Evaluación y Fomento de la Investigación), CIBERSAM (P82, 11INT3), and SENY Fundació. PC is supported by the Researcher Stabilization Program of the Health Department of the Generalitat de Catalunya. LK was recipient of a predoctoral fellowship from the Ministry of Science and Education. MR is recipient of an IDIBAPS fellowship. We acknowledge Noemí Jurado for skillful technical assistance. Also, Dr Toth (Cornell Univ) and Dr Gingrich are gratefully acknowledged by the supply of 5-HT1A and 5-HT2A receptor knockout mice, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr F Artigas has received consulting and educational honoraria from Eli Lilly, Sanofi-Aventis, Lundbeck, Pierre Fabre, and Elan. The rest of authors declare no conflict of interest related directly or indirectly to this work.
Rights and permissions
About this article
Cite this article
Kargieman, L., Riga, M., Artigas, F. et al. Clozapine Reverses Phencyclidine-Induced Desynchronization of Prefrontal Cortex through a 5-HT1A Receptor-Dependent Mechanism. Neuropsychopharmacol 37, 723–733 (2012). https://doi.org/10.1038/npp.2011.249
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2011.249
Keywords
This article is cited by
-
In vivo electrophysiological recordings of the effects of antidepressant drugs
Experimental Brain Research (2019)
-
Altered cortico-striatal crosstalk underlies object recognition memory deficits in the sub-chronic phencyclidine model of schizophrenia
Brain Structure and Function (2017)
-
Serotonin (5-HT)1A receptor agonism and 5-HT7 receptor antagonism ameliorate the subchronic phencyclidine-induced deficit in executive functioning in mice
Psychopharmacology (2016)
-
Examination of clozapine and haloperidol in improving ketamine-induced deficits in an incremental repeated acquisition procedure in BALB/c mice
Psychopharmacology (2016)
-
Clozapine-Induced Cardiotoxicity: Role of Oxidative Stress, Tumour Necrosis Factor Alpha and NF-κβ
Cardiovascular Toxicology (2015)