Abstract
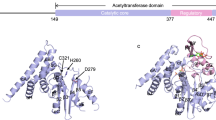
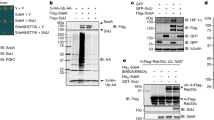
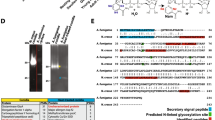
The Yersinia outer protein J (YopJ) family of bacterial effectors depends on a novel acetyltransferase domain to acetylate signalling proteins from plant and animal hosts. However, the underlying mechanism is unclear. Here, we report the crystal structures of PopP2, a YopJ effector produced by the plant pathogen Ralstonia solanacearum, in complex with inositol hexaphosphate (InsP6), acetyl-coenzyme A (AcCoA) and/or substrate Resistance to Ralstonia solanacearum 1 (RRS1-R)WRKY. PopP2 recognizes the WRKYGQK motif of RRS1-RWRKY to position a targeted lysine in the active site for acetylation. Importantly, the PopP2–RRS1-RWRKY association is allosterically regulated by InsP6 binding, suggesting a previously unidentified role of the eukaryote-specific cofactor in substrate interaction. Furthermore, we provide evidence for the reaction intermediate of PopP2-mediated acetylation, an acetyl-cysteine covalent adduct, lending direct support to the ‘ping-pong’-like catalytic mechanism proposed for YopJ effectors. Our study provides critical mechanistic insights into the virulence activity of YopJ class of acetyltransferases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zipfel, C. Plant pattern-recognition receptors. Trends Immunol. 35, 345–351 (2014).
Böhm, H., Albert, I., Fan, L., Reinhard, A. & Nürnberger, T. Immune receptor complexes at the plant cell surface. Curr. Opin. Plant Biol. 20, 47–54 (2014).
Jones, J. D. G. & Dangl, J. L. The plant immune system. Nature 444, 323–329 (2006).
Chisholm, S. T., Coaker, G., Day, B. & Staskawicz, B. J. Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124, 803–814 (2006).
Dean, P. Functional domains and motifs of bacterial type III effector proteins and their roles in infection. FEMS Microbiol. Rev. 35, 1100–1125 (2011).
Büttner, D. Behind the lines–actions of bacterial type III effector proteins in plant cells. FEMS Microbiol. Rev. 6, 894–937 (2016).
Costa, T. R. D. et al. Secretion systems in Gram-negative bacteria: structural and mechanistic insights. Nat. Rev. Micro. 13, 343–359 (2015).
Ma, K.-W. & Ma, W. Yopj family effectors promote bacterial infection through a unique acetyltransferase activity. Microbiol. Mol. Biol. Rev. 80, 1011–1027 (2016).
Lewis, J. D. et al. The YopJ superfamily in plant-associated bacteria. Mol. Plant. Pathol. 12, 928–937 (2011).
Jones, R. M. et al. Salmonella AvrA coordinates suppression of host immune and apoptotic defenses via JNK pathway blockade. Cell Host Microbe 3, 233–244 (2008).
Mittal, R., Peak-Chew, S. Y. & McMahon, H. T. Acetylation of MEK2 and IκB (IKK) activation loop residues by YopJ inhibits signaling. Proc. Natl Acad. Sci. USA 103, 18574–18579 (2006).
Mukherjee, S. et al. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 312, 1211–1214 (2006).
Paquette, N. et al. Serine/threonine acetylation of TGFβ-activated kinase (TAK1) by Yersinia pestis YopJ inhibits innate immune signaling. Proc. Natl Acad. Sci. USA 109, 12710–12715 (2012).
Trosky, J. E. et al. Vopa inhibits ATP binding by acetylating the catalytic loop of MAPK kinases. J. Biol. Chem. 282, 34299–34305 (2007).
Le Roux, C. et al. A receptor pair with an integrated decoy converts pathogen disabling of transcription factors to immunity. Cell 161, 1074–1088 (2015).
Sarris, P. F. et al. A plant immune receptor detects pathogen effectors that target WRKY transcription factors. Cell 161, 1089–1100 (2015).
Lee, A. H.-Y. et al. A bacterial acetyltransferase destroys plant microtubule networks and blocks secretion. PLoS Pathog. 8, e1002523 (2012).
Jiang, S. et al. Bacterial effector activates jasmonate signaling by directly targeting JAZ transcriptional repressors. PLoS Pathog. 9, e1003715 (2013).
Orth, K. et al. Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protein protease. Science 290, 1594–1597 (2000).
Zhang, Z.-M. et al. Structure of a pathogen effector reveals the enzymatic mechanism of a novel acetyltransferase family. Nat. Struct. Mol. Biol. 23, 847–852 (2016).
Ma, K.-W. et al. Two serine residues in Pseudomonas syringae effector HopZ1a are required for acetyltransferase activity and association with the host co-factor. New Phytol. 1157–1168 (2015).
Cheong, M. S. et al. Avrbst acetylates Arabidopsis ACIP1, a protein that associates with microtubules and is required for immunity. PLoS Pathog. 10, e1003952 (2014).
Mittal, R., Peak-Chew, S. Y., Sade, R. S., Vallis, Y. & McMahon, H. T. The acetyltransferase activity of the bacterial toxin YopJ of Yersinia is activated by eukaryotic host cell inositol hexakisphosphate. J. Biol. Chem. 285, 19927–19934 (2010).
Mukherjee, S., Hao, Y.-H. & Orth, K. A newly discovered post-translational modification – the acetylation of serine and threonine residues. Trends Biochem. Sci. 32, 210–216 (2007).
Duan, M.-R. et al. DNA binding mechanism revealed by high resolution crystal structure of Arabidopsis thaliana WRKY1 protein. Nucleic Acids Res. 35, 1145–1154 (2007).
Tasset, C. et al. Autoacetylation of the Ralstonia solanacearum effector PopP2 targets a lysine residue essential for RRS1-R-mediated immunity in Arabidopsis. PLoS Pathog. 6, e1001202 (2010).
Guttman, D. S. & Greenberg, J. T. Functional analysis of the type III effectors AvrRpt2 and AvrRpm1 of Pseudomonas syringae with the use of a single-copy genomic integration system. Mol. Plant Microbe Interact. 14, 145–155 (2001).
Yamasaki, K. et al. Structural basis for sequence-specific DNA recognition by an Arabidopsis WRKY transcription factor. J. Biol. Chem. 287, 7683–7691 (2012).
Morgan, R. L. et al. Catalytic domain of the diversified Pseudomonas syringae type III effector HopZ1 determines the allelic specificity in plant hosts. Mol. Microbiol. 76, 437–455 (2010).
Mossessova, E. & Lima, C. D. Ulp1-SUMO crystal structure and genetic analysis reveal conserved interactions and a regulatory element essential for cell growth in yeast. Mol. Cell 5, 865–876 (2000).
Pruneda, J. N. et al. The molecular basis for ubiquitin and ubiquitin-like specificities in bacterial effector proteases. Mol. Cell 63, 261–276 (2016).
Vetting, M. W. et al. Structure and functions of the GNAT superfamily of acetyltransferases. Arch. Biochem. Biophys. 433, 212–226 (2005).
Utley, R. T. & Côté, J. The MYST family of histone acetyltransferases. Curr. Top. Microbiol. Immunol. 274, 203–236 (2003).
Berndsen, C. E., Albaugh, B. N., Tan, S. & Denu, J. M. Catalytic mechanism of a MYST family histone acetyltransferase. Biochem 46, 623–629 (2007).
Cleland, W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 63, 103–138 (1979).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Kabsch, W . XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 (2002).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Case, D. A. et al. AMBER 2016 (Univ. California, 2016).
Rossato, G., Ernst, B., Smiesko, M., Spreafico, M. & Vedani, A. Probing small-molecule binding to cytochrome P450 2D6 and 2C9: an in silico protocol for generating toxicity alerts. ChemMedChem 5, 2088–2101 (2010).
Peters, M. B. et al. Structural survey of zinc-containing proteins and development of the zinc AMBER force field (ZAFF). J. Chem. Theory Comput. 6, 2935–2947 (2010).
Roe, D. R. & Cheatham, T. E. PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 9, 3084–3095 (2013).
Acknowledgements
This work was supported by NIH (1R35GM119721), Kimmel Scholar Award from Sidney Kimmel Foundation for Cancer Research and March of Dimes Foundation (1-FY15-345) to J.S., and grants from US NSF (IOS no. 0847870) and the USDA Agriculture Experimental Station Funding (CA-R-PPA-5075-H) to W.M. We would like to thank staff members at the Advanced Light Source, Lawrence Berkeley National Laboratory and Advanced Photon Source, Argonne National Laboratory for access to X-ray beamlines.
Author information
Authors and Affiliations
Contributions
Z-M.Z. and L.G. determined the crystal structures of PopP2 complexes and conducted ITC assays. J.S. and L.G. performed NMR analysis. K-W.M. and S.S. performed in vitro acetylation assays and in vivo functional analyses. Z.H. performed computational analysis. W.M. and J.S. designed and organized the study, Z.-M.Z., K-W.M., W.M. and J.S. prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interest.
Supplementary information
Supplementary Information
Supplementary Figures 1–9, Supplementary Tables 1 and 2. (PDF 2153 kb)
Rights and permissions
About this article
Cite this article
Zhang, ZM., Ma, KW., Gao, L. et al. Mechanism of host substrate acetylation by a YopJ family effector. Nature Plants 3, 17115 (2017). https://doi.org/10.1038/nplants.2017.115
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2017.115
This article is cited by
-
Secondary-structure switch regulates the substrate binding of a YopJ family acetyltransferase
Nature Communications (2021)
-
NOD-like receptor-mediated plant immunity: from structure to cell death
Nature Reviews Immunology (2021)
-
In-silico structural analysis of Pseudomonas syringae effector HopZ3 reveals ligand binding activity and virulence function
Journal of Plant Research (2021)
-
Bacterial virulence mediated by orthogonal post-translational modification
Nature Chemical Biology (2020)
-
Advancement of research on plant NLRs evolution, biochemical activity, structural association, and engineering
Planta (2020)