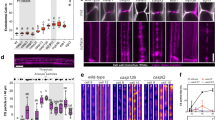
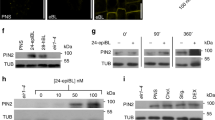
Abstract
Growth in plants depends on ion transport for osmotic solute uptake and secretory membrane trafficking to deliver material for wall remodelling and cell expansion. The coordination of these processes lies at the heart of the question, unresolved for more than a century, of how plants regulate cell volume and turgor. Here we report that the SNARE protein SYP121 (SYR1/PEN1), which mediates vesicle fusion at the Arabidopsis plasma membrane, binds the voltage sensor domains (VSDs) of K+ channels to confer a voltage dependence on secretory traffic in parallel with K+ uptake. VSD binding enhances secretion in vivo subject to voltage, and mutations affecting VSD conformation alter binding and secretion in parallel with channel gating, net K+ concentration, osmotic content and growth. These results demonstrate a new and unexpected mechanism for secretory control, in which a subset of plant SNAREs commandeer K+ channel VSDs to coordinate membrane trafficking with K+ uptake for growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zonia, L. & Munnik, T. Life under pressure: hydrostatic pressure in cell growth and function. Trends Plant Sci. 12, 90–97 (2007).
Walter, A., Silk, W. K. & Schurr, U. Environmental effects on spatial and temporal patterns in leaf and root growth. Annu. Rev. Plant Biol. 60, 279–304 (2009).
Orlowski, J. & Grinstein, S. Emerging roles of alkali cation/proton exchangers in organellar homeostasis. Curr. Opin. Cell Biol. 19, 483–492 (2007).
Amtmann, A. & Blatt, M. R. Regulation of macronutrient transport. New Phytol. 181, 35–52 (2009).
Grefen, C., Honsbein, A. & Blatt, M. R. Ion transport, membrane traffic and cellular volume control. Curr. Opin. Cell Biol. 14, 332–339 (2011).
Scheible, W. R. et al. An Arabidopsis mutant resistant to thaxtomin A, a cellulose synthesis inhibitor from Streptomyces species. Plant Cell 15, 1781–1794 (2003).
Geelen, D. et al. The abscisic acid-related SNARE homolog NtSyr1 contributes to secretion and growth: evidence from competition with its cytosolic domain. Plant Cell 14, 387–406 (2002).
Eisenach, C., Chen, Z. H., Grefen, C. & Blatt, M. R. The trafficking protein SYP121 of Arabidopsis connects programmed stomatal closure and K+ channel activity with vegetative growth. Plant J. 69, 241–251 (2012).
Blatt, M. R. Cellular signaling and volume control in stomatal movements in plants. Annu. Rev. Cell Dev. Biol. 16, 221–241 (2000).
Galvan-Ampudia, C. S. & Testerink, C. Salt stress signals shape the plant root. Curr. Opin. Cell Biol. 14, 296–302 (2011).
Campanoni, P. & Blatt, M. R. Membrane trafficking and polar growth in root hairs and pollen tubes. J. Exp. Bot. 58, 65–74 (2007).
Lipka, V., Kwon, C. & Panstruga, R. SNARE-Ware: the role of SNARE-Domain proteins in plant biology. Annu. Rev. Cell Dev. Biol. 23, 147–174 (2007).
Jahn, R. & Scheller, R. H. SNAREs - engines for membrane fusion. Nature Rev. Mol. Cell Biol. 7, 631–643 (2006).
Grefen, C. & Blatt, M. R. SNAREs - molecular governors in signalling and development. Curr. Opin. Cell Biol. 11, 600–609 (2008).
Leyman, B., Geelen, D., Quintero, F. J. & Blatt, M. R. A tobacco syntaxin with a role in hormonal control of guard cell ion channels. Science 283, 537–540 (1999).
Collins, N. C. et al. SNARE-protein-mediated disease resistance at the plant cell wall. Nature 425, 973–977 (2003).
Blatt, M. R., Grabov, A., Brearley, J., HammondKosack, K. & Jones, J. D. G. K+ channels of Cf-9 transgenic tobacco guard cells as targets for Cladosporium fulvum Avr9 elicitor-dependent signal transduction. Plant J. 19, 453–462 (1999).
Dangl, J. L. & Jones, J. D. G. Plant pathogens and integrated defence responses to infection. Nature 411, 826–833 (2001).
Honsbein, A., Blatt, M. R. & Grefen, C. A molecular framework for coupling cellular volume and osmotic solute transport control. J. Exp. Bot. 62, 2363–2370 (2011).
Honsbein, A. et al. A tripartite SNARE-K+ channel complex mediates in channel-dependent K+ nutrition in Arabidopsis. Plant Cell 21, 2859–2877 (2009).
Grefen, C. et al. A novel motif essential for SNARE interaction with the K+ channel KC1 and channel gating in Arabidopsis. Plant Cell 22, 3076–3092 (2010).
Sanderfoot, A. Increases in the number of SNARE genes parallels the rise of multicellularity among the green plants. Plant Physiol. 144, 6–17 (2007).
Dreyer, I. & Blatt, M. R. What makes a gate? The ins and outs of Kv-like K+ channels in plants. Trends Plant Sci. 14, 383–390 (2009).
Grefen, C. & Blatt, M. R. A 2in1 cloning system enables ratiometric bimolecular fluorescence complementation (rBiFC). Biotechniques 53, 311–314 (2012).
Chakrapani, S., Cuello, L. G., Cortes, D. M. & Perozo, E. Structural dynamics of an isolated voltage-sensor domain in a lipid bilayer. Structure 16, 398–409 (2008).
Palovcak, E., Delemotte, L., Klein, M. L. & Carnevale, V. Evolutionary imprint of activation: the design principles of VSDs. J. Gen. Physiol. 143, 145–156 (2014).
Richter, S., Voss, U. & Juergens, G. Post-Golgi traffic in plants. Traffic 10, 819–828, (2009).
Tyrrell, M. et al. Selective targeting of plasma membrane and tonoplast traffic by inhibitory (dominant-negative) SNARE fragments. Plant J. 51, 1099–1115 (2007).
Karnik, R. et al. Arabidopsis Sec1/Munc18 protein sec11 is a competitive and dynamic modulator of SNARE binding and SYP121-dependent vesicle traffic. Plant Cell 25, 1368–1382 (2013).
Hecker, A. et al. Binary 2in1 vectors improve in planta (co-) localisation and dynamic protein interaction studies. Plant Physiol. 168,776–787 (2015).
Sutter, J. U., Campanoni, P., Tyrrell, M. & Blatt, M. R. Selective mobility and sensitivity to SNAREs is exhibited by the Arabidopsis KAT1 K+ channel at the plasma membrane. Plant Cell 18, 935–954 (2006).
Karnik, R. et al. Binding of SEC11 indicates its role in SNARE recycling after vesicle fusion and identifies two pathways for vesicular traffic to the plasma membrane. Plant Cell 27, 675–694 (2015).
Assaad, F. F. et al. The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol. Biol. Cell 15, 5118–5129 (2004).
Zhang, Z. G. et al. A SNARE-protein has opposing functions in penetration resistance and defence signalling pathways. Plant J. 49, 302–312 (2007).
Rehman, R. U. et al. Tomato Rab11a characterization evidenced a difference between SYP121-dependent and SYP122-dependent exocytosis. Plant Cell Physiol. 49, 751–766 (2008).
Fujiwara, M. et al. Interactomics of Qa-SNARE in Arabidopsis thaliana. Plant Cell Physiol. 55, 781–789 (2014).
Latorre, R. et al. Molecular coupling between voltage sensor and pore opening in the Arabidopsis inward rectifier K+ channel KAT1. J. Gen. Physiol. 122, 459–469 (2003).
Lai, H. C., Grabe, M., Jan, Y. N. & Jan, L. Y. The S4 voltage sensor packs against the pore domain in the KAT1 voltage-gated potassium channel. Neuron 47, 395–406 (2005).
Lefoulon, C. et al. Voltage-sensor transitions of the inward-rectifying K+ channel KAT1 indicate a closed-to-open latching mechanism that is biased by hydration around the S4 alpha-helix. Plant Physiol. 166, 960–975, (2014).
Tsutsui, H., Karasawa, S., Okamura, Y. & Miyawaki, A. Improving membrane voltage measurements using FRET with new fluorescent proteins. Nature Methods 5, 683–685 (2008).
Youvan, D. C. et al. Calibration of fluorescence resonance energy transfer in microscopy using genetically engineered GFP derivatives on nickel chelating beads. Biotechnology 3, 1–18 (1997).
Chen, Z. H., Grefen, C., Donald, N., Hills, A. & Blatt, M. R. A bicistronic, Ubiquitin-10 promoter-based vector cassette for transient transformation and functional analysis of membrane transport demonstrates the utility of quantitative voltage clamp studies on intact Arabidopsis root epidermis. Plant Cell Environ. 34, 554–564 (2011).
Fricker, M. D., Runions, J. & Moore, I. Quantitative fluorescence microscopy: from art to science. Ann. Rev. Plant Biol. 57, 79–107 (2006).
Sokolovski, S., Hills, A., Gay, R. & Blatt, M. R. Functional interaction of the SNARE protein NtSyp121 in Ca2+ channel gating, Ca2+ transients and ABA signalling of stomatal guard cells. Mol. Plant 1, 347–358 (2008).
Wang, Y., Hills, A. & Blatt, M. R. Systems analysis of guard cell membrane transport for enhanced stomatal dynamics and water use efficiency. Plant Physiology 164, 1593–1599 (2014).
Wang, Y. et al. Overexpression of plasma membrane H+-ATPase in guard cells promotes light-induced stomatal opening and enhances plant growth. Proc. Natl Acad. Sci. USA 111, 533–538 (2014).
Zhang, B. et al. The R-SNARE VAMP721 interacts with KAT1 and KC1 K+ channels to moderate K+ current at the plasma membrane. Plant Cell 27, 1697–1717 (2015).
Leung, Y. M., Kwan, E. P., Ng, B., Kang, Y. & Gaisano, H. Y. SNAREing voltage-gated K+ and ATP-Sensitive K+ channels Tuning beta-Cell excitability with syntaxin-1A and other exocytotic proteins. Endocrine Rev. 28, 653–663 (2007).
Iwasaki, H. et al. A voltage-sensing phosphatase, Ci-VSP, which shares sequence identity with PTEN, dephosphorylates phosphatidylinositol 4,5-bisphosphate. Proc. Natl Acad. Sci. USA 105, 7970–7975 (2008).
Tombola, F., Ulbrich, M. H. & Isacoff, E. Y. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron 58, 546–556 (2008).
Lawson, T. & Blatt, M. R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 164, 1556–1570 (2014).
Grefen, C. et al. A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. Plant J. 64, 355–365 (2010).
Karnik, A., Karnik, R. & Grefen, C. SDM-Assist software to design site-directed mutagenesis primers introducing “silent” restriction sites. BMC Bioinformatics 14, 105 (2013).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Grefen, C. & Blatt, M. R. Do calcineurin B-like proteins interact independently of the serine threonine kinase CIPK23 with the K+ channel AKT1? Lessons learned from a menage a trois. Plant Physiol. 159, 915–919 (2012).
Blatt, M. R. & Grefen, C. in Arabidopsis Protocols Vol. 3 (eds Sanchez-Serrano, J. J. & Salinas, J. ) 487–507 (2014).
Acknowledgements
We are grateful to N. Donald and L. Matas for technical support. G. Boswell and A. Ruiz-Pardo helped with Xenopus and plant maintenance. This work was supported by a Chinese Scholarship Council award to B.Z. and by grants BB/H0024867/1, BB/I024496/1, BB/K015893/1, BBL001276/1 and BB/M001601/1 from the UK Biotechnology and Biological Sciences Research Council to M.R.B. C.G. is supported by an Emmy Noether Fellowship from the Deutsche Forschungsgemeinschaft GR 4251/1-1.
Author information
Authors and Affiliations
Contributions
M.R.B. conceived and wrote the manuscript with C.G., R.K. and E.L.; C.G., designed and generated vectors; C.G., R.K., B.Z. and C.L. prepared constructs; C.G. and B.Z. carried out the split-ubiquitin studies; C.G., R.K. and E.L. generated the stable lines, and R.K. and E.L. analysed the lines; R.K. and S.W. carried out pulldown and immunoblot analysis; M.R.B. carried out the rBiFC, secretion and Mermaid FRET assays; C.L. recorded K+ currents in oocytes, Y.W. recorded K+ currents in Arabidopsis; A.H. developed analysis utilities for this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Grefen, C., Karnik, R., Larson, E. et al. A vesicle-trafficking protein commandeers Kv channel voltage sensors for voltage-dependent secretion. Nature Plants 1, 15108 (2015). https://doi.org/10.1038/nplants.2015.108
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2015.108
This article is cited by
-
VAMP726 and VAMP725 regulate vesicle secretion and pollen tube growth in Arabidopsis
Plant Cell Reports (2023)
-
Engineering a K+ channel ‘sensory antenna’ enhances stomatal kinetics, water use efficiency and photosynthesis
Nature Plants (2022)
-
Genome-wide identification and expression analysis of the SNARE genes in Foxtail millet (Setaria italica) reveals its roles in drought stress
Plant Growth Regulation (2021)
-
SNARE proteins and their role in plant ion channel regulation
Plant Growth Regulation (2020)
-
Growth and development: Close relations of secretion and K+
Nature Plants (2015)