Abstract
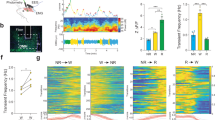
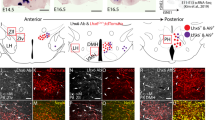
Epidermal growth factor receptor (EGFR) signaling in the mammalian hypothalamus is important in the circadian regulation of activity. We have examined the role of this pathway in the regulation of sleep in Drosophila melanogaster. Our results demonstrate that rhomboid (Rho)- and Star-mediated activation of EGFR and ERK signaling increases sleep in a dose-dependent manner, and that blockade of rhomboid (rho) expression in the nervous system decreases sleep. The requirement of rho for sleep localized to the pars intercerebralis, a part of the fly brain that is developmentally and functionally analogous to the hypothalamus in vertebrates. These results suggest that sleep and its regulation by EGFR signaling may be ancestral to insects and mammals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hendricks, J.C. et al. Rest in Drosophila is a sleep-like state. Neuron 25, 129–138 (2000).
Shaw, P.J., Cirelli, C., Greenspan, R.J. & Tononi, G. Correlates of sleep and waking in Drosophila melanogaster. Science 287, 1834–1837 (2000).
Andretic, R., van Swinderen, B. & Greenspan, R.J. Dopaminergic modulation of arousal in Drosophila. Curr. Biol. 15, 1165–1175 (2005).
Koh, K., Evans, J.M., Hendricks, J.C. & Sehgal, A. A Drosophila model for age-associated changes in sleep:wake cycles. Proc. Natl. Acad. Sci. USA 103, 13843–13847 (2006).
Kramer, A. et al. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science 294, 2511–2515 (2001).
Kushikata, T., Fang, J., Chen, Z., Wang, Y. & Krueger, J.M. Epidermal growth factor enhances spontaneous sleep in rabbits. Am. J. Physiol. 275, R509–R514 (1998).
Urban, S., Lee, J.R. & Freeman, M. A family of Rhomboid intramembrane proteases activates all Drosophila membrane-tethered EGF ligands. EMBO J. 21, 4277–4286 (2002).
Shilo, B.Z. Signaling by the Drosophila epidermal growth factor receptor pathway during development. Exp. Cell Res. 284, 140–149 (2003).
Shilo, B.Z. Regulating the dynamics of EGF receptor signaling in space and time. Development 132, 4017–4027 (2005).
Guichard, A. et al. Rhomboid and Star interact synergistically to promote EGFR/MAPK signaling during Drosophila wing vein development. Development 126, 2663–2676 (1999).
Brand, A.H. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
Sturtevant, M.A., Roark, M. & Bier, E. The Drosophila rhomboid gene mediates the localized formation of wing veins and interacts genetically with components of the EGFR signaling pathway. Genes Dev. 7, 961–973 (1993).
Schweitzer, R., Shaharabany, M., Seger, R. & Shilo, B.Z. Secreted Spitz triggers the DER signaling pathway and is a limiting component in embryonic ventral ectoderm determination. Genes Dev. 9, 1518–1529 (1995).
Freeman, M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell 87, 651–660 (1996).
Urban, S., Lee, J.R. & Freeman, M. Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell 107, 173–182 (2001).
Guichard, A., Srinivasan, S., Zimm, G. & Bier, E. A screen for dominant mutations applied to components in the Drosophila EGFR pathway. Proc. Natl. Acad. Sci. USA 99, 3752–3757 (2002).
McGuire, S.E., Le, P.T., Osborn, A.J., Matsumoto, K. & Davis, R.L. Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302, 1765–1768 (2003).
Sturtevant, M.A., O'Neill, J.W. & Bier, E. Down-regulation of Drosophila EGFR mRNA levels following hyperactivated receptor signaling. Development 120, 2593–2600 (1994).
Welsh, J.B., Gill, G.N., Rosenfeld, M.G. & Wells, A. A negative feedback loop attenuates EGF-induced morphological changes. J. Cell Biol. 114, 533–543 (1991).
Wiley, H.S. et al. The role of tyrosine kinase activity in endocytosis, compartmentation, and down-regulation of the epidermal growth factor receptor. J. Biol. Chem. 266, 11083–11094 (1991).
Ng, D.C. & Bogoyevitch, M.A. The mechanism of heat shock activation of ERK mitogen-activated protein kinases in the interleukin 3–dependent ProB cell line BaF3. J. Biol. Chem. 275, 40856–40866 (2000).
Williams, J.A., Su, H.S., Bernards, A., Field, J. & Sehgal, A. A circadian output in Drosophila mediated by neurofibromatosis-1 and Ras/MAPK. Science 293, 2251–2256 (2001).
Schejter, E.D., Segal, D., Glazer, L. & Shilo, B.Z. Alternative 5′ exons and tissue-specific expression of the Drosophila EGF receptor homolog transcripts. Cell 46, 1091–1101 (1986).
Botella, J.A. et al. Deregulation of the Egfr/Ras signaling pathway induces age-related brain degeneration in the Drosophila mutant vap. Mol. Biol. Cell 14, 241–250 (2003).
Joiner, W.J., Crocker, A., White, B.H. & Sehgal, A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature 441, 757–760 (2006).
Pitman, J.L., McGill, J.J., Keegan, K.P. & Allada, R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature 441, 753–756 (2006).
Rajashekhar, K.P. & Singh, R.N. Neuroarchitecture of the tritocerebrum of Drosophila melanogaster. J. Comp. Neurol. 349, 633–645 (1994).
Siegmund, T. & Korge, G. Innervation of the ring gland of Drosophila melanogaster. J. Comp. Neurol. 431, 481–491 (2001).
de Velasco, B. et al. Specification and development of the pars intercerebralis and pars lateralis, neurocndocrine command centers in the Drosophila brain. Dev. Biol. 302, 309–323 (2007).
Veelaert, D., Schoofs, L. & De Loof, A. Peptidergic control of the corpus cardiacum-corpora allata complex of locusts. Int. Rev. Cytol. 182, 249–302 (1998).
De Velasco, B., Shen, J., Go, S. & Hartenstein, V. Embryonic development of the Drosophila corpus cardiacum, a neuroendocrine gland with similarity to the vertebrate pituitary, is controlled by sine oculis and glass. Dev. Biol. 274, 280–294 (2004).
Kilduff, T.S. & Peyron, C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 23, 359–365 (2000).
Saper, C.B., Scammell, T.E. & Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 437, 1257–1263 (2005).
Mignot, E., Taheri, S. & Nishino, S. Sleeping with the hypothalamus: emerging therapeutic targets for sleep disorders. Nat. Neurosci. 5 Suppl, 1071–1075 (2002).
Saper, C.B., Chou, T.C. & Scammell, T.E. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 24, 726–731 (2001).
Yarden, Y. & Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2, 127–137 (2001).
Garcia, R.A., Vasudevan, K. & Buonanno, A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc. Natl. Acad. Sci. USA 97, 3596–3601 (2000).
Huang, Y.Z. et al. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron 26, 443–455 (2000).
Suzuki, T., Okumura-Noji, K. & Nishida, E. ERK2-type mitogen-activated protein kinase (MAPK) and its substrates in postsynaptic density fractions from the rat brain. Neurosci. Res. 22, 277–285 (1995).
Suzuki, T., Mitake, S. & Murata, S. Presence of upstream and downstream components of a mitogen-activated protein kinase pathway in the PSD of the rat forebrain. Brain Res. 840, 36–44 (1999).
Humbert, P., Russell, S. & Richardson, H. Dlg, Scribble and Lgl in cell polarity, cell proliferation and cancer. Bioessays 25, 542–553 (2003).
Hoeffer, C.A., Sanyal, S. & Ramaswami, M. Acute induction of conserved synaptic signaling pathways in Drosophila melanogaster. J. Neurosci. 23, 6362–6372 (2003).
Sweatt, J.D. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr. Opin. Neurobiol. 14, 311–317 (2004).
Schrader, L.A. et al. ERK/MAPK regulates the Kv4.2 potassium channel by direct phosphorylation of the pore-forming subunit. Am. J. Physiol. Cell Physiol. 290, C852–C861 (2006).
Cirelli, C. et al. Reduced sleep in Drosophila Shaker mutants. Nature 434, 1087–1092 (2005).
Shaw, P.J., Tononi, G., Greenspan, R.J. & Robinson, D.F. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature 417, 287–291 (2002).
Sokal, R.R. & Rohlf, F.J. Biometry: the Principles and Practice of Statistics in Biological Research (Freeman, New York, 1995).
Rosato, E. & Kyriacou, C.P. Analysis of locomotor activity rhythms in Drosophila. Nat. Protoc. 1, 559–568 (2006).
Roenneberg, T. & Taylor, W. Automated recordings of bioluminescence with special reference to the analysis of circadian rhythms. Methods Enzymol. 305, 104–119 (2000).
Basyuk, E., Bertrand, E. & Journot, L. Alkaline fixation drastically improves the signal of in situ hybridization. Nucleic Acids Res. 28, E46 (2000).
Acknowledgements
We dedicate this paper to the memory of our mentor, colleague and friend, John Newport, who died 25 December 2005. We thank A. Guichard for essential technical guidance and advice, E. J. Kim and J. Wagner for technical assistance, M. Kaneko, R. Andretic, H. Dierick, P. Shaw, C.P. Kyriacou, E. Green, T. Roenneberg and M. Merrow for assistance with data analysis, and E. Bier, J. Wang, W. McGinnis, R. Andretic, M. Kaneko and H. Dierick for comments on the manuscript. This work was supported by a US National Institutes of Health grant (J.W.N.) and a US National Science Foundation grant No. 0432063 (R.J.G.). R.J.G. is the Dorothy and Lewis B. Cullman Fellow at the Neurosciences Institute, which is supported by the Neurosciences Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2, Tables 1–3 (PDF 6035 kb)
Rights and permissions
About this article
Cite this article
Foltenyi, K., Greenspan, R. & Newport, J. Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nat Neurosci 10, 1160–1167 (2007). https://doi.org/10.1038/nn1957
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1957
This article is cited by
-
Novel pseudo-aspartic peptidase from the midgut of the tick Rhipicephalus microplus
Scientific Reports (2019)
-
Exploring phylogeny to find the function of sleep
Nature Reviews Neuroscience (2019)
-
JmjC domain proteins modulate circadian behaviors and sleep in Drosophila
Scientific Reports (2018)
-
Identifying pathways modulating sleep duration: from genomics to transcriptomics
Scientific Reports (2017)
-
Aberrant Axonal Arborization of PDF Neurons Induced by Aβ42-Mediated JNK Activation Underlies Sleep Disturbance in an Alzheimer’s Model
Molecular Neurobiology (2017)