Abstract
GABAergic neurons of the hypothalamus regulate many innate behaviors, but little is known about the mechanisms that control their development. We previously identified hypothalamic neurons that express the LIM homeodomain transcription factor Lhx6, a master regulator of cortical interneuron development, as sleep-promoting. In contrast to telencephalic interneurons, hypothalamic Lhx6 neurons do not undergo long-distance tangential migration and do not express cortical interneuronal markers such as Pvalb. Here, we show that Lhx6 is necessary for the survival of hypothalamic neurons. Dlx1/2, Nkx2-2, and Nkx2-1 are each required for specification of spatially distinct subsets of hypothalamic Lhx6 neurons, and that Nkx2-2+/Lhx6+ neurons of the zona incerta are responsive to sleep pressure. We further identify multiple neuropeptides that are enriched in spatially segregated subsets of hypothalamic Lhx6 neurons, and that are distinct from those seen in cortical neurons. These findings identify common and divergent molecular mechanisms by which Lhx6 controls the development of GABAergic neurons in the hypothalamus.
Similar content being viewed by others
Introduction
Although much is now known about both the diversity and development of GABAergic neurons of the telencephalon1,2, far less is known about their counterparts in the hypothalamus, where over 20% of neurons are GABAergic3. Previous work shows that hypothalamic GABAergic neuronal precursors first appear in a domain that separates the anterodorsal and posteroventral halves of the developing hypothalamus, and is delineated by expression of transcription factors that regulate the development of telencephalic GABAergic neurons, including Dlx1/2 and Arx4,5,6,7,8. Within this structure, which has been termed the intrahypothalamic diagonal/tuberomammillary terminal (ID/TT), nested expression domains of LIM homeodomain family genes are observed, in which expression of Lhx1, Lhx8, and Lhx6 delineates the anterior–posterior axis of the ID/TT4. Lhx1 is essential for the terminal differentiation and function of neurons in the master circadian oscillator in the suprachiasmatic nucleus9,10,11. Lhx6-expressing neurons in the zona incerta (ZI) of the hypothalamus are sleep-promoting and activated by elevated sleep pressure, and hypothalamic-specific loss of function of Lhx6 disrupts sleep homeostasis12.
Lhx6 has been extensively studied in the developing telencephalon. It is essential for the specification, migration, and maturation of GABAergic neurons of the telencephalon—particularly the cortex and hippocampus13,14. Lhx6 is expressed in the medial ganglionic eminence (MGE) of the embryonic telencephalon, where it is co-expressed with both Nkx2-1 and Dlx1/215,16,17. Shh induces expression of Nkx2-118, which in turn directly activates Lhx6 expression16,19. Nkx2-1, in turn, cooperates with Lhx6 to directly activate the expression of multiple other genes that control cortical interneuron specification and differentiation, including Sox6 and Gsx220,21. Furthermore, Lhx6 is both necessary and sufficient for the tangential migration of the great majority of interneuron precursors from the MGE to their final destinations in the cortex and hippocampus17,22,23. Finally, Lhx6 expression persists in mature interneurons that express parvalbumin (Pvalb) and somatostatin (Sst), and is necessary for their expression22.
The functional role of Lhx6 in hypothalamic development has not been previously investigated. However, previous studies imply this may differ in certain key ways from its function in the developing telencephalon. Notably, the hypothalamic domain of Lhx6 expression only partially overlaps with that of Nkx2-14. Furthermore, in sharp contrast to cortical interneurons, Lhx6 is not co-expressed with either Pvalb or Sst in the ZI12. In this study, we sought to determine the extent to which gene regulatory networks controlling the development of hypothalamic Lhx6 neurons diverge from those that control the development of telencephalic Lhx6 neurons. We find that hypothalamic Lhx6 regulates neuronal differentiation and survival. Further, we observe extensive molecular heterogeneity among mature hypothalamic Lhx6 neurons and a lack of overlap with annotated subtypes of Lhx6-expressing cortical interneurons. Combinatorial patterns of transcription factor expression delineate spatial subdomains of Lhx6 expression within the ID/TT, and we find that Nkx2-1, Nkx2-2, and Dlx1/2 each regulate expression of Lhx6 in largely nonoverlapping domains. Finally, Lhx6 neurons derived from Nkx2-2-expressing precursors are activated by sleep pressure. These findings identify mechanisms by which Lhx6 can regulate the development of hypothalamic GABAergic neurons, and more broadly, how diverse subtypes of hypothalamic neurons can be generated during development.
Results
Distribution of hypothalamic Lhx6-expressing neurons
Our previous work has indicated that Lhx6 is expressed in two continuous yet distinct domains of the developing hypothalamus: the intrahypothalamic diagonal (ID) and the more posterior tuberomammillary terminal (TT)4,5. We next sought to more carefully determine the expression pattern of Lhx6 and its putative regulators during early hypothalamic development. High-quality chromogenic in situ hybridization (ISH) detects both the ID and TT domain of Lhx6 expression at E11.5, E12.5, and E14.5 (Fig. 1A–E). By E16.5, hypothalamic Lhx6-expressing neurons are observed in the ZI and dorsomedial hypothalamus (DMH), in a pattern that broadly corresponds to the earlier ID domain, while expression in the posterior hypothalamus (PH) in turn broadly corresponds to the TT domain (Fig. 1F, G). This closely matches the pattern of hypothalamic Lhx6 expression previously reported in adults12. Lhx6-expressing neurons are only a small minority of hypothalamic GABAergic neurons12, with single-cell RNA-sequencing (scRNA-Seq) revealing that only ~2% of all hypothalamic GABAergic neuronal precursors (defined by Gad1/2 and Dlx1/2 expression) express Lhx6 between E11 and E13 (Fig. 1H)24.
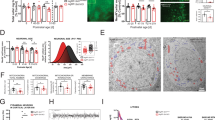
A, B, E–G In situ hybridization showing Lhx6 (blue) and Shh (brown) at E11.5 (A), E12.5 (B), E14.5 (E), shown in sagittal planes, and E16.5 (F, G) in coronal planes. Red arrows (A, B, E) indicate the intrahypothalamic diagonal (ID), red arrowheads (A, B, E) indicate the tuberomammillary terminal (TT), black arrows (D) indicate migrated telencephalic Lhx6-expressing cells (tangential migration from the medial ganglionic eminence to the cortex). Red arrows in (F) indicate the ZI and red circle in (F) indicates the DMH, and red arrows in (G) indicate the PH. C, D Schematics showing the distribution of telencephalic (green) and hypothalamic (purple) Lhx6-expressing cells at E11.5, with ID (red) and TT (blue) are highlighted in (D) (E11.5) Note anterior domains to the ID that shows a weak and transient Lhx6 expression during development (Ant.ID anterior ID, DMH dorsomedial hypothalamus). H scRNA-Seq from E11-E13 hypothalamus scRNA-Seq from24 showing the distribution of neurons that express GABAergic markers (blue, Dlx1/2, Gad1/2, and Slc32a1) and Lhx6-expressing GABAergic neurons (brown) that are ~2% of all hypothalamic GABAergic neurons during development. I Schematic distribution of Lhx6-expressing neurons across the dorsolateral hypothalamus (red = neurons that continue to express Lhx6, purple = neurons that transiently expressed Lhx6) across ZIv (zona incerta ventral), ZIl (zona incerta lateral), LH (lateral hypothalamus), DMH (dorsomedial hypothalamus), VMH (ventromedial hypothalamus), and PH (posterior hypothalamus). J–C′ Lhx6-antibody staining (gray), tdTomato expression from Lhx6Cre/+;;Ai9 line (red), NeuN (yellow) in ZIv (J–M), ZIl (N, Q), DMH (R–U), LH (V–Y), PH (Z–C′). L = lateral, M = medial. White arrowheads show neurons that transiently expressed Lhx6, and white arrows show neurons continue to express Lhx6). D′ A bar graph showing the percentage of tdTomato+ and Lhx6-expressing neurons over the total number of tdTomato+ neurons. Scale bar = 0.45 mm (A), 0.5 mm (B, F, G), 0.55 mm (E), 0.1 mm (I–C′). All bar graphs (D′) show mean and standard error of the mean (SEM), with individual data points plotted.
This regional pattern of hypothalamic Lhx6 expression is broadly similar to that reported for Lhx6Cre/+;Ai9 mice (Fig. 1J–D′)12, with ~65–70% of tdTomato-expressing neurons in the ZI, DMH, and PH of Lhx6Cre/+;Ai9 postnatal mice also continuing to express Lhx6 (Fig. 1J–U, D′). Notably, we see a few tdTomato-expressing neurons in other hypothalamic regions, with the largest numbers found in adjacent structures such as the ventromedial hypothalamus (VMH) and lateral hypothalamus (LH), although only ~5% of these tdTomato-expressing neurons still express Lhx6 (Fig. 1V–D′). This shows that, in contrast to telencephalic interneuron precursors, hypothalamic Lhx6 cells do not appear to undergo long-distance tangential migration, and that hypothalamic Lhx6-expressing cells that do undergo short-range tangential dispersal during early development generally repress Lhx6 expression as they mature.
Lhx6 is necessary for the survival of hypothalamic neurons
These findings led us to investigate other potential differences in the Lhx6 function in hypothalamic neurons relative to the telencephalon. While Lhx6 does not regulate the survival of cortical interneuron precursors22, hypothalamic-specific loss of function of Lhx6 leads to substantial changes in sleep patterns12, raising the possibility that Lhx6 may be necessary for the viability or proper functions of these neurons.
To investigate this possibility, we tested P8 Lhx6CreER/CreER mice, in which a CreER cassette has been inserted in frame with the start codon to generate a null mutant of Lhx6, to determine if read-through transcription of endogenous Lhx6 could be detected in the hypothalamus (Fig. 2A). Chromogenic ISH of telencephalic structures such as the amygdala and cortex reveals that Lhx6-expressing cells are still detected in both regions, although the number of Lhx6-expressing cells in the cortex is substantially reduced in Lhx6CreER/CreER mice relative to Lhx6CreER/+ heterozygous controls (Fig. 2B–M). This is consistent with the severe reduction in tangential migration of cortical interneurons seen in Lhx6-deficient mice22,23. In the hypothalamus, however, no read-through transcription of Lhx6 was detected (Fig. 2J, M). This implies that, in contrast to its role in the telencephalon where Lhx6 is necessary for the tangential migration and proper laminar positioning21,23, hypothalamic Lhx6 is required to promote neuronal survival and/or to activate its expression.
A Lhx6CreER knock-in site (JAX #010776) and location of the Lhx6 probe used to detect read-through transcription. B–M Coronal planes showing Lhx6 mRNA expression in the zona incerta (ZI, B, C, H, I), amygdala (AMY, F, G, L, M), and cortex (CTX, D, E, J, K) in control (Lhx6CreER/+, B–G) and mutant (Lhx6CreER/CreER, H–M) at P8. Note Lhx6 mRNA is not detected in the Lhx6-deficient hypothalamus but is detected in the telencephalon. N Schematic diagram showing the overall design of the experiment from three genotypes (1. Lhx6CreER/+;Ai9, 2. Lhx6CreER/lox;Ai9, 3. Lhx6CreER/lox;;Baxlox/lox;Ai9). O Schematic diagram showing the overall outcome of the experiment. P A bar graph showing the percentage of tdTomato+/(tdTomato+ and tdTomato+/Lhx6+) across three genotypes in five different brain regions. *Indicates p < 0.05. DMH dorsomedial hypothalamus, PH posterior hypothalamus. Q–Y Representative images of three genotypes (1. Lhx6CreER/+;Ai9 (Q–S), 2. Lhx6CreER/lox;Ai9 (T–V), 3. Lhx6CreER/lox;Baxlox/lox;Ai9 (W–Y) in ZI. More images of different brain regions are available in Supplementary Fig. 1. Z Potential candidate genes from bulk RNA-Seq (Supplementary Fig. 2) controlling cell survival can be regulated by Lhx6 in hypothalamic Lhx6+ neurons: Neuregulin-ErbB4 signaling and Gdnf signaling pathways (Supplementary Figs. S3, S4). Scale bar = 0.6 mm (B–M), 100 μm (Q–Y). All bar graphs (P) show mean and standard error of the mean (SEM), with individual data points plotted.
To distinguish between these possibilities, we sought to determine whether neonatal loss of function of Lhx6 would lead to the death of Lhx6-expressing neurons. This was done using the genetic fate mapping of Lhx6-deficient neurons. Using a series of 4-Hydroxytamoxifen (4-OHT) injections between postnatal day (P) 1 and P5 in Lhx6CreER/+Ai9 and Lhx6CreER/lox;Ai9 mice, we labeled Lhx6-expressing cells with tdTomato while also simultaneously disrupting Lhx6 function in a subset of Lhx6-expressing neurons in Lhx6CreER/lox mice (Fig. 2N). We then quantified the number of neurons that expressed both tdTomato and Lhx6 protein at P45, as well as the number of neurons that only expressed tdTomato. Expression of the only tdTomato indicates that a cell has lost expression of Lhx6, either as a result of Cre-dependent disruption of the Lhx6 locus or as a result of normal repression of expression during postnatal development (Fig. 2O). In both hypothalamic and telencephalic regions in Lhx6CreER/+;Ai9 mice, we observed that the fraction of neurons that only express tdTomato was only 10–15% of the number of neurons expressing both Lhx6 and tdTomato (Fig. 2P–S, Supplementary Fig. 1). This indicates that the great majority of neurons in both regions that express Lhx6 in neonates continue to do so at P45. However, when we performed this same analysis in Lhx6CreER/lox;Ai9 mice, we found that while 75% of tdTomato-expressing neurons in the cortex and amygdala remain even in the absence of detectable Lhx6 protein, a substantially smaller fraction of tdTomato-expressing neurons are detected in the absence of Lhx6 protein in the ZI, DMH, and PH (Fig. 2P, T–V, Supplementary Fig. 1).
This is consistent with Lhx6 playing a selective role in regulating the survival of Lhx6-expressing hypothalamic neurons. To directly address this hypothesis, we next generated Lhx6CreER/lox;Baxlox/lox;Ai9 mice, with loss of function of Bax predicted to selectively prevent apoptosis in Lhx6-expressing neurons25. When Cre recombinase activity was induced using the same protocol, we observed that the fraction of tdTomato-expressing neurons that lacked Lhx6 expression was indistinguishable from that seen in cortex and amygdala (Fig. 2P, W–Y, Supplementary Fig. 1).
These data indicate that Lhx6 is selectively required for the survival of hypothalamic Lhx6-expressing neurons. To determine whether Lhx6 is also required for normal differentiation of these cells, we next conducted RNA-Seq analysis on sorted tdTomato-expressing hypothalamic cells from P10 Lhx6CreER/+;Ai9 and Lhx6CreER/lox;Baxlox/lox;Ai9 mice (Fig. 2Z, Supplementary Fig. 2). We observe that Lhx6CreER/lox;Baxlox/lox;Ai9 mice show no change in expression of markers of GABAergic neurons, including Gad1, Gad2, Slc32a1. However, substantially increased expression of genes expressed in mitotic neural progenitors, including Ccna1, Aurka, Msx1, and Msx2 (Supplementary Fig. 2, Table S1), is observed, along with a decreased expression of axon guidance/growth factors such as Sema3c, Sema4d, and Sema5a. Notably, we also observe ectopic expression of genes that are not normally found in the brain but are expressed in germline stem cells (Sycp1), testes (Ccdc144b, Samd15, Stag3) mucosa (Slc12a8), colon (Nlrp6), liver (Tfr2), heart (Popdc2, Spta1), and cochlear hair cells (Pdzd7)26. This suggests that, as in telencephalic neurons, Lhx6 is not required for expression of GABAergic markers21,22,23, but might be required to repress inappropriate expression of genes expressed both in neural progenitor and in nonneuronal cells. This does not, however, exclude the possibility that these may be in part induced as a result of the loss of function of Bax.
Genetic and biochemical analyses have identified several genes as direct or indirect Lhx6 targets in the developing telencephalon18,19,21,23,27. These include Shh, the transcription factors Arx, Cux2, Mafb, and Nkx2-1; as well as Sst and chemokine receptors such as Cxcr4, Cxcr7, and Erbb4. To identify genes and signaling pathways that are strong candidates for selectively regulating survival of hypothalamic Lhx6 neurons, the bulk RNA-Seq data from P10 Lhx6CreER/+;Ai9 neurons were directly compared to profiles obtained from FACS-isolated Lhx6-GFP positive and negative hypothalamic and cortical neurons that were collected at E15.5, P8 (Fig. 2Z, Supplementary Fig. 2), since regulation of hypothalamic Lhx6 in cell survival is evident during embryonic and early neonatal periods and we expected to detect potential signaling pathways at both datasets. Genes found to be enriched in hypothalamic samples of bulk RNA-Seq data were then compared to scRNA-Seq datasets of hypothalamic Lhx6-expressing neurons collected at E15.5 and P8 (Fig. 2Z, Supplementary Fig. 2)24, and a core set of Lhx6-regulated genes that were selectively enriched in hypothalamic Lhx6-expressing neurons was thus identified.
We observe that many previously identified Lhx6 targets either show little detectable expression in wildtype hypothalamic Lhx6 neurons, (Cux2, Mafb, Sst, and Cxcr4/7) or else showed no detectable change in expression following Lhx6 loss of function (Arx, Nkx2-1). One notable exception is the Neuregulin receptor Erbb4, which has been shown to be necessary for tangential migration and differentiation of MGE-derived immature Lhx6-expressing cortical interneurons28,29,30. Erbb4 is both highly expressed in hypothalamic Lhx6 neurons, and its expression is strongly Lhx6-dependent (Fig. 2Z, Supplementary Fig. 2). Since Neuregulin signaling is also neurotrophic in many cell types31, this suggested that the loss of neuregulin signaling could be a potential mechanism behind the apoptotic death of Lhx6-deficient hypothalamic cells. Indeed, we observed that additional components of the both the Neuregulin (Nrg1) and Gdnf (Ret, Gfra1, Gfra2) neurotrophic signaling pathways were selectively enriched in hypothalamic Lhx6 neurons (Fig. 2Z, Supplementary Fig. 2), a finding which was confirmed using fluorescent ISH and scRNA-Seq (Supplementary Fig. 3).
Diverse subtypes of Lhx6-expressing neurons are found in the postnatal hypothalamus
Our previous work12 showed that adult ZI Lhx6-expressing neurons do not highly express traditional markers of MGE Lhx6+ derived GABAergic neurons of the cortex. No ZI Lhx6-expressing neurons co-express Pvalb and Sst, with only a small subset expressing Npy12. We thus hypothesized that subtypes of Lhx6 neurons in the postnatal hypothalamus might be diverged substantially from those present in the cortex32, and might be more molecularly heterogeneous.
scRNA-Seq analysis of P8 Lhx6-eGFP neurons from the hypothalamus that expressed high levels of Lhx6 mRNA shows that these neurons express a diverse pool of neuropeptides and neurotransmitters that are not expressed in telencephalic Lhx6-expressing neurons, including Gal, and Trh (Fig. 3, Supplementary Fig. 4, Table S2). Other markers that are specific to distinct subsets of cortical Lhx6 neurons were expressed in hypothalamic Lhx6 neurons, such as Pnoc, Tac1, Nos1, and Th. Hypothalamic Lhx6-expressing neurons do not express Pvalb, but a small fraction expresses Npy and Cck. We also identified a rare subpopulation of hypothalamic Lhx6-expressing neurons in the PH that co-express Sst, although these are absent in more anterior regions (Fig. 3, Supplementary Fig. 4). Tac1 is expressed broadly in cortical and hypothalamic Lhx6-expressing neurons. Similar patterns of gene expression are observed in scRNA-Seq data obtained from Lhx6 neurons in the adult hypothalamus of mice that are older than P30 (Supplementary Fig. 5)24,33,34. However, all these enriched markers (neuropeptides and neurotransmitters) are not specific to Lhx6-expressing neurons but rather expressed broadly in hypothalamic GABAergic neurons across nuclei (Supplementary Fig. 6).
A Uniform Manifold Approximation and Projection (UMAP) plot showing hypothalamic Lhx6-expressing GABAergic neurons at P8 scRNA-Seq. B Violin plots showing key markers in individual clusters in (A). C UMAP plot showing Lhx6-expressing neurons originated from ID and TT. D UMAP plots showing the distribution of diverse neuropeptides and neurotransmitters across ID and TT derived Lhx6-expressing neurons. E–P Fluorescent in situ hybridization showing Lhx6-GFP (gray) with Calb1 (red, E–H), Calb2 (red, I–L), and Gal (red, M–P). Scale bar = 50 μm.
Mature Lhx6 hypothalamic neurons were organized into three major clusters that showed close similarity to the two subdomains of the ID and the main TT region observed at E12.5, and in turn appear to represent individual subtypes of Lhx6 neurons that are differentially distributed along the anteroposterior axis of the hypothalamus, and which may correspond to Lhx6 neurons of the ZI, DMH, and PH, respectively (Fig. 3, Supplementary Fig. 4). Lhx6 neurons express a mixture of Pnoc, Penk, Calb1, Calb2, Cck in both the ZI and DMH, whereas Tac1 is more restricted to the ZI. Npy and Nos1 are enriched in DMH Lhx6 neurons. Th, Trh, Gal are located in the region spanning the DMH and PH, while Sst is expressed only in a small subset of PH Lhx6 neurons.
scRNA-Seq identifies molecular markers of spatially distinct domains of hypothalamic Lhx6 neurons
Lhx6-expressing neurons of the postnatal hypothalamus are molecularly diverse and distributed across a broad region of the dorsolateral hypothalamus. We hypothesized that this diversity is regulated by multiple transcription factors that control the specification of region-specific subtypes of Lhx6-expressing neurons.
To identify these anatomically and molecularly distinct Lhx6-expressing domains in the hypothalamus, we performed scRNA-Seq with the Lhx6-GFP line at E12.5 and E15.5. At E12.5 and E15.5, scRNA-Seq analysis readily distinguishes the ID, TT, and hinge domains (Fig. 4A, Supplementary Figs. 7, 8). By E12.5, all Lhx6 cells in the hypothalamus express the early neuronal precursor marker Dcx, as well as the synaptic GABA transporter Slc32a1, but do not express progenitor markers (e.g., Fabp7 and Ascl1). It is not immediately clear whether the molecular identities of anatomically and molecularly distinct clusters of Lhx6-expressing cells in the ID, TT and hinge clusters are already distinct at E12.5, we used RNA velocity analysis35 to determine whether any cells appeared to be undergoing transition between individual clusters. RNA velocity analysis does not identify trajectories connecting individual clusters, indicating that their regional identity appears to be fixed by this age (Fig. 4B, C). In addition, weak Lhx6 expression was observed in Lhx1 and Lhx8 co-expressing neurons of the anterior ID cluster, which are Nkx2-1+ (Fig. 4D, E), and give rise to GABAergic neurons in the suprachiasmatic nucleus and DMH, although little or no Lhx6 mRNA was detected in these neurons after E13.5 (Figs. 1, 6)4,10. We observed that Dlx1/2, Nkx2-2, and Nkx2-1 are differentially expressed in the ID, hinge, and TT domains, respectively, at both ages (Fig. 4D, E, Supplementary Fig. 8). These three transcription factors are each shown as key putative regulatory transcription factors of the ID, hinge, and TT domains respectively (Fig. 4F). Furthermore, we observe several molecularly distinct cell clusters that have not been previously described. The first cluster expresses low levels of Nkx2-1, but high levels of Prox1 and Sp9, transcription factors that are highly expressed in the developing prethalamus. This may therefore correspond to a dorsal subdomain of the TT located adjacent to the hinge domain (Fig. 4D, E). We also observe a distinction between more proximal and distal domains of the ID, based on the expression of Nefl, Dlx6, Nefm, Lhx1, and Nr2f1.
A UMAP plot showing different Lhx6-expressing hypothalamic regions at E12.5. B UMAP plots showing a lack of expression of Fabp7 (proliferating cells), Ascl1 (proneural), whereas the neuronal precursor marker Dcx and the GABAergic neuronal marker Slc32a1 is highly expressed. C UMAP plot with RNA velocity trajectories. D UMAP plots showing expression and percentage of ID, hinge, and TT Lhx6 neurons expressing Dlx1/2, Nkx2-2, and Nkx2-1. E Violin plots showing expression of key transcription factors (and other genes) that are highly expressed in individual domains. F A heatmap showing z-scores of significantly differentially expressed key regulatory transcription factors among Lhx6+ hypothalamic regions. Note activity of Dlx1 in the ID, Nkx2-2 in the hinge, and Nkx2-1 in the TT. Ant.ID anterior ID, DMH dorsomedial hypothalamus.
In all, five molecularly distinct clusters of neurons that strongly express Lhx6 could be resolved in the embryonic hypothalamus (Fig. 4). These can be distinguished not only by the expression of different subsets of transcription factors at E12.5, but also by more conventional markers of cell identity such as neuropeptides and calcium-binding proteins such as Sst, Tac1, Pnoc, Islr2, Gal, and Npy at E15.5 (Supplementary Fig. 8, Tables S3, S4). We also observed clusters that were located in the hinge and TT region at E12.5 (Supplementary Fig. 8 cluster 4 and 7), but which postnatally expressed markers that are restricted to neurons at the most anterior domain of hypothalamic Lhx6 neurons. These markers include Nfix, Nfib, and Tcf4 (Supplementary Fig. 8, Fig. 3, Tables S2–S4).
These molecularly distinct domains of hypothalamic Lhx6 neurons were also visualized using traditional two-color ISH with Nkx2-1, Nkx2-2, Arx, and Prox1 probes (Supplementary Fig. 9). This also confirms that Shh is only expressed in dorsal TT Lhx6 neurons, while Six3 is expressed only in the weakly Lhx6-expressing neurons in the anterior ID. scRNA-Seq showed that Lef1, which is expressed broadly in the ID and TT region at E12.5, was expressed in only very few Lhx6 neurons at both E12.5 and E15.5 (Fig. 4, Supplementary Fig. 9), indicating that Lef1 and Lhx6 are not extensively co-expressed.
Dlx1/2, Nkx2-2, and Nkx2-1 mediate patterning of discrete spatial domains of hypothalamic Lhx6 neurons
Dlx1/2, Nkx2-2, and Nkx2-1 are selectively expressed in the ID, hinge, and TT domains, respectively. Since these three transcription factors were also identified as putative key regulatory transcription factors from scRNA-Seq analysis, we sought to investigate their function in regulating Lhx6 expression in more detail. Using Lhx6-GFP mice, which faithfully recapitulate the endogenous expression pattern of Lhx612, we integrated bulk RNA-Seq analysis obtained at E15.5 and P0 from hypothalamus with age-matched ATAC-Seq data to cross-reference our scRNA-Seq result (Fig. 5A). We further sought to investigate similarities and differences in gene expression and chromatin accessibility in age-matched hypothalamic and telencephalic Lhx6-expressing neurons (Fig. 5), since the role of Lhx6 in development of telencephalic interneurons is extensively studied, and it is therefore critically important to connect these findings to prior work characterizing Lhx6 mechanisms of action in forebrain development.
A Schematic showing bulk RNA-Seq and bulk ATAC-Seq pipelines from flow-sorted Lhx6-GFP+ neurons of the cortex and hypothalamus at E15.5 and P0. B Peaks in ATAC-Seq showing potential transcription factor binding sites near the promoter regions of differentially expressed genes from bulk RNA-Seq data in the cortex and hypothalamus at E15.5 and P0. C Schematic showing the ID/TT of the developing hypothalamus and expression of Dlx1/2 (left), and the percentage of ID Lhx6-expressing neurons that co-express Dlx1 (right). D–G Immunostaining with Lhx6 (purple) and Dlx1 (green) of E13.5 hypothalamus, showing co-localization of Lhx6 and Dlx1 in the ID of the hypothalamus (G, red arrows). H Schematic showing ID/TT of the developing hypothalamus and expression of Nkx2-2 (left) and the percentage of hinge Lhx6-expressing neurons that co-express Nkx2-2 (right). I–L Immunostaining with Lhx6 (purple) and GFP in Nkx2-2CreGFP/+ (green) of E13.5 hypothalamus show co-localization of Lhx6 and Nkx2-2GFP between the ID and TT (hinge region) of the hypothalamus (L, red arrows). M Schematic showing the ID/TT of the developing hypothalamus and expression of Nkx2-1 (left) and the percentage TT Lhx6-expressing neurons that co-express Nkx2-1 (right). N–Q Immunostaining with Lhx6 (purple) and Nkx2-1 (green) of E13.5 hypothalamus, showing co-localization of Lhx6 and Dlx1 in the ID of the hypothalamus (Q, red arrows). Scale bar = 50 μm.
At E15, many region-specific differences in gene expression were observed between hypothalamic and telencephalic Lhx6-expressing neurons, particularly for transcription factors. We observed enriched expression of Six3, Nkx2-2, and Nkx2-4 in the hypothalamus. As predicted by earlier studies, we observed enriched expression of the telencephalic marker Foxg1, Satb2, and Nr2e136,37, in the cortex (Fig. 5B, Table S5).
However, expression of genes broadly expressed in GABAergic neurons showed no significant differences, including Nkx2-1, and Dlx1/2. At P0, hypothalamic Lhx6 neurons continued to show enriched expression for multiple transcription factors, including Prox1, Foxp2, and Nhlh2. Hypothalamic Lhx6 neurons show little detectable expression of the cortical interneuron markers Pvalb, Sst, and Npy, but we observed a higher level of Gal and Pnoc at P0 in hypothalamic Lhx6 neurons. Relative to Lhx6-negative hypothalamic neurons, we also observed a higher level of transcription factors such as Dlx1, Onecut1, Pax5, and Nkx2-2 in hypothalamic Lhx6 neurons compare to the rest of the hypothalamus at E15.5, as well as a higher level of Tac1 and Pnoc at P0 (Supplementary Fig. 10, Table S6).
Regions of accessible chromatin identified by ATAC-Seq were, as expected, clustered in the proximal promoter and intronic regions of annotated genes in all samples profiled (Supplementary Fig. 10, Tables S7, S8). Region-specific differences in chromatin accessibility frequently corresponded to differences in mRNA expression. For instance, proximal promoter and/or intronic regions of Foxg1, Npy, Pvalb, and Sst were selectively accessible in cortical Lhx6 neurons, while those of Nkx2-2, Sall3, and Gal were accessible only in the hypothalamus at both E15.5 and P0 (Fig. 5B, Tables S7, S8, Supplementary Fig. 10). However, substantial differences in chromatin accessibility were also observed for Nkx2-1 and Dlx1/2 at both E15.5 and P0, implying that different gene regulatory networks may control the expression of these genes in hypothalamus and cortex (Tables S7, S8).
To determine whether any of the spatial domains of Lhx6 expression could closely resemble telencephalic Lhx6 cells, we compared E12.5 hypothalamic scRNA-Seq results to data previously obtained from E13.5 MGE38. These data confirmed that, while transcription factors such as Nkx2-1, Dlx1/2, and Lhx8 are broadly expressed in Lhx6 MGE cells, they are not expressed (Lhx8) or expressed only in discrete subsets (Nkx2-1, and Dlx1/2) of hypothalamic Lhx6 neurons. No identified subset of hypothalamic Lhx6 neurons resembled MGE Lhx6 cells (Supplementary Fig. 11A, B, Table S9).
With substantial differences between hypothalamic and telencephalic Lhx6-expressing neurons in both gene expression and chromatin accessibility, we reasoned that the transcriptional regulatory networks identified as controlling the development of telencephalic Lhx6-expressing neurons would not broadly apply in developing hypothalamus. Thus, based on both scRNA-Seq data and analysis of our ATAC-Seq data, as well as our previous work4,24, three previously mentioned transcription factors—Nkx2-1, Dlx1/2, and Nkx2-2—emerged as strong candidates for regulating specific domains of hypothalamic Lhx6 neurons. Nkx2-1 is required for Lhx6 expression in the telencephalon16,19 and is expressed in the TT, but not ID, domain in the hypothalamus4,24, while Dlx1/2 are required for tangential migration of cortical interneurons and are also broadly expressed in both cortical and hypothalamic Lhx6 neurons4,6,8,39. Nkx2-2, in contrast, is expressed only in the hypothalamus in a zone immediately dorsal to the region of Nkx2-1 expression4,40.
Each of these transcription factors is expressed in discrete spatial domains that overlap with distinct subsets of hypothalamic Lhx6 neurons at E13.5 (Fig. 5C–Q). Dlx1 was strongly expressed in the ID (Fig. 5C–G, Supplementary Fig. 11C–H), but not the TT. Nkx2-2, in contrast, selectively demarcated the region joining the ID and TT (Fig. 5H–L), which we have termed the hinge domain. Nkx2-1 was selectively expressed in the TT region, but essentially absent from the ID and hinge domain (Fig. 5M–Q, Supplementary Fig. 11I–K). These spatial differences in the expression of Dlx1 and Nkx2-1 in hypothalamic Lhx6 neurons are preserved at E17.5, where Dlx1 is enriched in the more anterior ZI and DMH (Supplementary Fig. 12), and Nkx2-1 expression is enriched in the PH (Supplementary Fig. 12A–L). Furthermore, unlike the MGE Lhx6-expressing cells, Dlx1 and Nkx2-1 formed mutually exclusive expression domains in the ID and TT (Supplementary Fig. 11F–K). However, we observed a much more even distribution of Nkx2-2/Lhx6 neurons across the ZI, DMH, and PH, which could indicate either short-range tangential dispersal of hinge neurons or widespread induction of Nkx2-2 expression in Lhx6 neurons at later ages (Supplementary Fig. 12M–X). These results indicate that distinct spatial domains of hypothalamic Lhx6 expression can be delineated by combinatorial patterns of homeodomain transcription factor expression.
To determine the final location of Nkx2-1 expressing Lhx6 neurons, we next used fate-mapping analysis, in which Nkx2-1CreER/+;Ai9 mice41 were labeled with 4-OHT at E11 (Supplementary Fig. 13). At E18, tdTomato expression was detected in the majority of Lhx6-expressing neurons in the amygdala and cortex (Supplementary Fig. 13) as expected16,19, but we observed anterior–posterior bias in the distribution of tdTomato-expressing neurons in the hypothalamus that closely matched the location of Lhx6/Nkx2-1 expressing neurons at earlier ages. We observe that only a small fraction (~10%) of ZI Lhx6-expressing neurons, which correspond to the most anterior region of Lhx6 expression at later developmental ages12, were labeled with tdTomato. In contrast, a much larger fraction of PH Lhx6 neurons, corresponding to the most posterior domain of Lhx6 expression, were tdTomato positive. This implies that Nkx2-1/Lhx6-expressing neurons of the TT primarily give rise to Lhx6 neurons found in the PH, but that a small fraction may undergo tangential migration to more anterior structures such as the ZI. This was also shown with immunostaining of Nkx2-1 and Lhx6-expressing neurons at E17.5 (Supplementary Fig. 12Y-J′).
We next investigated whether loss of function of Nkx2-1, Nkx2-2, and Dlx1/2 led to the loss of spatially-restricted hypothalamic expression of Lhx6. We first examined Nkx2-1CreER/CreER mice, in which targeted insertion of the CreER cassette generates a null mutation in Nkx2-141. This leads to severe hypoplasia of the posteroventral hypothalamus, as previously reported for targeted Nkx2-1 null mutants42. The ventrally-extending TT domain of Lhx6 expression is not detected in Nkx2-1-deficient mice at E12.5, but the Nkx2-1-negative ID domain persists (Fig. 6, Supplementary Fig. 14). Fate-mapping analysis, in which Nkx2-1CreER/+;Ai9 and Nkx2-1CreER/CreER;Ai9 mice were injected with tamoxifen at E11 and analyzed at E18, indicate that surviving Lhx6 neurons in the ID region represent a mixture of tdTomato-positive and -negative neurons, and confirm that a subset of these surviving neurons derived from Nkx2-1-expressing precursors. As previously reported, no Lhx6-expressing neurons are detected in the mutant cortex (Supplementary Fig. 14).
A–E, L RNAscope showing Lhx6 expression (magenta) in control (A), Nkx2-1CreER/CreER (B), Foxd1Cre/+:Dlx1/2lox/lox (C), and Nkx2-2CreGFP/CreGFP (D, E). Pixel density of Lhx6 is shown across all four groups in (L). F–I, M Nkx2-1CreER/CreER;Ai9 (4-OHT treatment at E11.5, collection at E18.5) showing Lhx6 expression (gray) and tdTomato (red) in the hypothalamus. Arrows in (I) indicate Lhx6+ and tdTomato+ neurons. Raw number of Lhx6+ or tdTomato+ (tdT) neurons (left) and percentage of Lhx6+ and tdTomato+ neurons (right) in Nkx2-1CreER/+;Ai9 (Supplementary Fig. 5) and Nkx2-1CreER/CreER/Ai9. J, K, N Lhx6 expression (magenta) in control and Dlx1/2−/− at E12 ID and TT. The number of Lhx6-expressing neurons in the ID and TT are shown in (N). O Schematic diagram showing distribution of Lhx6 expression across four groups. ID intrahypothalamic diagonal, TT tuberomamillary terminal. Scale bar = 50 μm. ***p < 0.05. All bar graphs (L, M, N) show mean and standard error of the mean (SEM), with individual data points plotted.
We next generated null mutants of Nkx2-2 in the same manner, generating mice homozygous for a knock-in CreGFP cassette that disrupts expression of the endogenous Nkx2-2 locus43. In this case, we observe a loss of Lhx6 expression in the hinge region, located between the posterior ID and dorsal TT (Fig. 6, Supplementary Fig. 14). Finally, we examined the phenotype of mice deficient for Dlx1/2, examining both global knockouts44 and Foxd1Cre/+;Dlx1/2lox/lox mutants45, in which Dlx1/2 are selectively deleted in hypothalamic and prethalamic neuroepithelium12,46,47. In both global and diencephalic-specific Dlx1/2 knockouts, the ID domain of Lhx6 expression is absent at E12.5, whereas the TT domain is intact. At E17, we also observe a major reduction in the number of Lhx6-expressing neurons in the ZI (Supplementary Fig. 14). These results indicate that spatially discrete domains of hypothalamic Lhx6 expression are controlled by the expression of different transcription factors (Supplementary Fig. 14).
Nkx2.2-derived Lhx6-expressing neurons in the ZI respond to sleep pressure
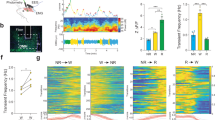
Our previous work showed that around 40% of ZI Lhx6-expressing neurons respond to sleep pressure, and ZI Lhx6 neurons promote both REM and NREM sleep12. We sought to identify whether Lhx6 neurons derived from Nkx2.2-expressing precursors might selectively respond to sleep pressure. Nkx2-2 is uniquely expressed in hypothalamic Lhx6 neurons, but Nkx2-2 is absent in cortical Lhx6 neurons, unlike Nkx2-1 and Dlx1/2. Our scRNA-Seq analysis and immunostaining indicate that a small number of Nkx2-2+ Lhx6-expressing neurons are located in the postnatal ZI (Supplementary Fig. 14). In addition, RNA velocity analysis on the combined E12.5 and E15.5 scRNA-Seq datasets to identify potential lineage relationships between individual clusters at E12.5 and E15.5 indicates that cells in the Nkx2-2+ cluster in E15.5 are derived from both cells located in the ID and hinge region, indicating potential short-range tangential migration from the hinge region to the ID, which in turn leads to a subset of Nkx2-2+ Lhx6-expressing neurons reaching the ZI (Fig. 7). This is supported by our observation that 28% of Lhx6 ZI neurons express Nkx2.2 at E17.5 (Supplementary Fig. 12).
A UMAP plot with RNA velocity trajectories of E12.5 and E15.5 combined scRNA-Seq dataset. B UMAP plot shows distinct domains of hypothalamic Lhx6 neurons. A specific population that continues to express the transcription factor Nkx2-2 is derived from the ID and dorsal hinge region. C–F TdTomato expression in Nkx2-2Cre/+;;Ai9 mice (red), and Lhx6-antibody staining (gray) identifies Lhx6 neurons in the zona incerta (ZI) are derived from Nkx2-2-expressing precursors. G A bar graph showing the percentage of tdTomato+ and Lhx6-expressing neurons relative to the total number of Lhx6-expressing neurons in the ZI, dorsomedial hypothalamus (DMH, Supplementary Fig. 15) and posterior hypothalamus (PH, Supplementary Fig. 15). H–P GFP expression from Lhx6-GFP (green, H–P), cFos antibody staining (gray) and Nkx2-2 antibody staining (red) shows a specific population of Nkx2-2+ Lhx6 neurons in ZI that selectively responds to sleep pressure. L UMAP plot showing Nkx2-2 expression in the anterior portion of Lhx6 neurons. Q A bar graph showing the percentage of cFos+ and Lhx6-GFP+ neurons relative to the total number of Lhx6-GFP+ neurons, and demonstrates that a subset of sleep pressure-responsive Lhx6 neurons express Nkx2-2. R A bar graph showing the percentage of cFos+ and Nkx2-2+ neurons relative to the total number of Nkx2-2+ neurons, and that a subset of sleep-pressure responding Lhx6 neurons express Nkx2-2. Scale bar = 100 μm. All bar graphs (G, Q, R) show mean and standard error of the mean (SEM), with individual data points plotted.
To determine what fraction of Lhx6-expressing ZI neurons express Nkx2.2 during development, we performed lineage-tracing analysis using Nkx2-2Cre/+;Ai9 mice, analyzing the distribution of TdTom/Lhx6-expressing neurons at P45 (Fig. 7, Supplementary Fig. 14), and observed that ~30% of Lhx6 ZI neurons co-labeled with tdTomato, along with a similar fraction of Lhx6 DMH neurons. In contrast, only a small fraction (~5%) of PH Lhx6-expressing neurons were labeled with tdTomato.
ScRNA-Seq analysis and immunostaining reveal that 30% of Lhx6-expressing ZI neurons continue to express Nkx2.2 in adulthood, raising the question of their potential physiological function. We next then performed 6 h sleep-deprivation, a robust method to detect cells that respond to sleep pressure, on Lhx6-GFP mice12 and stained with antibodies to Nkx2-2 and cFos. As shown previously, around 40% of Lhx6-expressing neurons in the ZI responded to sleep pressure, and around 35% of sleep pressure-activated neurons (~15% of all Lhx6-expressing neurons in the ZI) were Nkx2-2+ (Fig. 7). In total, 25% of Nkx2-2+ ZI neurons express cFos in response to increased sleep pressure. This indicates that Nkx2.2 may guide the differentiation of a distinct subset of sleep-promoting ZI neurons.
Discussion
The LIM homeodomain factor Lhx6 is a master regulator of the differentiation and migration of GABAergic neurons of the cortex and hippocampus, as well as many other subcortical telencephalic structures such as striatum and amygdala. Over 70% of cortical interneurons express Lhx6 into adulthood, where it is required for expression of canonical markers of interneuron subtype identity such as Sst and Pvalb27,32. In contrast, Lhx6 is expressed in only 1–2% of hypothalamic GABAergic neurons. Lhx6 expression is confined to a broad domain in the dorsolateral hypothalamus, and Lhx6-expressing cells do not undergo widespread long-distance tangential migration. Lhx6-expressing hypothalamic neurons in the ZI play an essential role in promoting sleep12, but their function is otherwise uncharacterized. In this study, we seek to characterize the development and molecular identity of hypothalamic Lhx6-expressing neurons, using previous knowledge obtained from studying telencephalic Lhx6-expressing neurons.
In the hypothalamus, in sharp contrast to the telencephalon, Lhx6 is required to prevent neuronal apoptosis (Supplementary Fig. 16). The fact that loss of function of hypothalamic Lhx6 leads to death of sleep-promoting neurons in the ZI may account for the more severe changes in sleep pattern that is seen in the hypothalamic-specific loss of function of Lhx6 than is observed following DREADD-based manipulation of the activity of these neurons12. Analysis of Lhx6/Bax double mutants identified both the Neuregulin and Gdnf signaling pathways as potential neurotrophic mechanisms that promote the survival of hypothalamic Lhx6 neurons. Interestingly, Nrg1/Erbb4-dependent signaling acts as a chemorepellent signal, while Gdnf signaling acts as a chemoattractant, and both regulate the long-range tangential migration of cortical Lhx6 neurons29,48. Both signaling pathways may therefore have been at least partially repurposed to regulate cell survival in hypothalamic Lhx6 neurons. The more modest phenotype seen following postnatal loss of function of Lhx6, relative to the constitutive mutant, may indicate that the survival of a specific subset of Lhx6-expressing neurons is no longer Lhx6-dependent at later ages.
We observe extensive transcriptional divergence between developing telencephalic and hypothalamic Lhx6 neurons. Notably, we observe clear spatial differences in gene expression among hypothalamic Lhx6 neurons that are not detectable in the MGE. While MGE cells require Nkx2-1 to activate Lhx6 expression, Nkx2-1 is expressed primarily in the TT, in the posterior domain of hypothalamic Lhx6 expression. The TT domain also expresses Shh similar to MGE that may regulate Nkx2-1 expression18,49, leading to activation of Lhx6 expression. However, we fail to observe any upstream gene expression (Shh or Nkx2-1) in MGE scRNA-Seq clusters when the downstream gene is detected (Nkx2-1 or Lhx6)38, indicating Nkx2-1 and Lhx6 activation could lead to a shutdown of Shh and Nkx2-1 in the MGE. In our hypothalamic Lhx6-expressing neurons, all three genes (Shh, Nkx2-1, and Lhx6) are highly co-expressed in the TT domain, unlike in the MGE.
Dlx1/2 are expressed in virtually all Lhx6-expressing MGE cells but are not required to maintain Lhx6 expression19,21, while Dlx1/2 is primarily expressed in the ID domain in the hypothalamus. Furthermore, Nkx2-2 is not expressed in the telencephalon but is selectively expressed in a previously uncharacterized hinge domain that connects the ID and TT. We find that mutants in Nkx2-1, Nkx2-2, and Dlx1/2 selectively eliminate hypothalamic Lhx6 expression in the TT, hinge, and ID domains, respectively. This indicates a high level of spatial patterning and transcriptional diversity among developing hypothalamic Lhx6 neurons. Although hypothalamic Lhx6 neurons do not undergo extensive tangential dispersal, as observed in telencephalon, lineage analysis indicates that by E18, a subset of neurons that express the TT-specific marker Nkx2-1 have migrated to anterior structures such as the ZI. Combined with the observation that Nkx2-2-derived Lhx6 neurons progressively disperse from the hinge domain into the ID implies that subsets of hypothalamic Lhx6 neurons may undergo short-range migration during development.
Lhx6 neurons in the postnatal hypothalamus are likewise highly transcriptionally diverse and do not directly correspond to any of their telencephalic counterparts (Supplementary Fig. 16). No hypothalamic Lhx6 neurons express Pvalb, and only a few selected subsets express either Sst or Npy. In the cortex, many genes are exclusively expressed in Lhx6-expressing neurons—including Sst, Pvalb, and Npy. In contrast, in the hypothalamus, no genes were identified that were exclusively expressed in Lhx6 neurons, other than Lhx6 itself. Neuropeptides such as Pnoc, which are expressed in large subsets of hypothalamic Lhx6 neurons, are also widely expressed in many neurons that do not express Lhx6. Finally, molecularly distinct subtypes of Lhx6 neurons are broadly and evenly distributed in the cortex, owing to the widespread tangential dispersal during development. In contrast, in the hypothalamus, we observe clear differences in the expression of neuropeptides and calcium-binding proteins in Lhx6 neurons that broadly correspond to the spatial position of these neurons.
These results provide a starting point to not only better define the molecular mechanisms that control differentiation, survival, and diversification of hypothalamic Lhx6 neurons, but also serve as a molecular toolbox for selectively targeting molecularly distinct neuronal subtypes. Previous studies identified Lhx6 neurons of the ZI as being unique in promoting both NREM and REM sleep12. Identification of molecular markers that distinguish different subtypes of Lhx6 neurons in this region can help determine whether this is produced by the activation of distinct neuronal subtypes. We demonstrate that not only are a substantial fraction of Lhx6 ZI neurons derived from Nkx2.2-expressing precursors, but that many also continue to express Nkx2.2 into adulthood (Supplementary Fig. 16). Indeed, Nkx2-2+ Lhx6-expressing ZI neurons represent 25% of Lhx6 ZI neurons that express c-fos in response to elevated sleep pressure. Hypothalamic Lhx6 neurons also send and receive connections from many brain regions that regulate innate behaviors, including the amygdala, periaqueductal gray, and ventral tegmental area12. The function of these circuits is as yet unknown, and the molecular markers identified in this study can serve as a starting point for investigating their behavioral significance.
Methods
Mice
All experimental animal procedures were approved by the Johns Hopkins University Institutional Animal Care and Use Committee. All mice were housed in a climate-controlled facility (14 h dark and 10 h light cycle) with ad libitum access to food and water.
Lhx6-GFP (Tg(Lhx6-EGFP)BP221Gsat)50, Lhx6CreER knock-in (B6(Cg)-Lhx6tm1(cre/ERT2)Zjh/J, JAX #010776)41, Lhx6lox/lox51, Ai9 (B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J, JAX #007909)52, Baxlox/lox (B6;129-Baxtm2Sjk Bak1tm1Thsn/J, JAX #006329)25, Nkx2-2CreGFP (B6.129S6(Cg)-Nkx2-2tm4.1(cre/EGFP)Suss/J, JAX #026880)43, Nkx2-1CreER (Nkx2-1tm1.1(cre/ERT2)Zjh/J, JAX #014552)41, Foxd1Cre (B6;129S4-Foxd1tm1(GFP/cre)Amc/J, JAX #012463)53, Dlx1/2lox/lox (Dlx1tm1Rth Dlx2tm1.1Rth/J, JAX #025612)45, Dlx1/2−/− (gift from J.L.R.R.) were used. Mice were time-mated and embryos at various ages (embryonic day (E)11.5, E12.5, E13.5, E15.5, E16.5, E17.5, E18.5, and postnatal day (P) 8) were collected for high-throughput sequencing and histology. Day of birth was considered as P0.
Tamoxifen injection
Lhx6CreER pulse-chase experiments
Pups with parental crosses of Lhx6CreER/+;Lhx6lox/+;Baxlox/+;Ai9 (Lhx6CreER/lox;Baxlox/+;Ai9) × Lhx6CreER/+;Lhx6lox/+;Baxlox/+;Ai9 (Lhx6CreER/lox;Baxlox/+;Ai9) were treated with intraperitoneal 4-Hydroxytamoxifen injection (4-OHT, 0.5 mg/per day, in corn-oil) for 5 consecutive days between P1 and P5. Pups were genotyped on the day of the birth and three different genotypes (1. Lhx6CreER/+;Ai9, 2. Lhx6CreER/lox;Ai9, 3. Lhx6CreER/lox;Baxlox/lox;Ai9) were used. Lhx6CreER/CreER genotype dies soon after weaning41, Lhx6CreER/+;Lhx6lox/lox genotype is not possible to generate due to similar sites of CreER and lox insertion.
Treated pups were collected between P40 and P45 and processed as described below. Cell counting was conducted in all three genotypes in the ZI, DMH, PH, S1 somatosensory cortex (CTX), and amygdala (AMY) following the Mouse Brain Atlas54. Borders were drawn to separate individual regions, using DAPI counterstaining and the Mouse Brain Atlas as a guideline, and 6500 μm × 500 μm region-of-interest was used to count across cortical layers per section. Three sections (every second section to avoid counting the same cell) were used per region, and six brains that were collected from between two and three individual litters (different parents) were used. tdTomato expression was observed in blood vessels as previously described12.
Three different classes of neurons were counted. The first class consists of neurons that only express Lhx6 protein as detected by immunostaining (indicating that no 4-OHT-induced Cre recombination occurred at the Lhx6 locus). The second class consists of neurons that expressed the only tdTomato but not Lhx6 (indicating Cre-mediated activation of tdTomato, and disruption of Lhx6). The third class consists of neurons that expressed both tdTomato and Lhx6 (indicating incomplete 4-OHT-induced Cre recombination, with the induction of tdTomato expression and failure to recombine the conditional allele of Lhx6). Only neurons that expressed tdTomato (with or without Lhx6 protein expression) were counted and the total counted the number of neurons used as a denominator. Neurons that only expressed tdTomato were used as a numerator to calculate cell survival rate, as we expect to observe a decrease in the ratio (tdTomato+/(tdTomato+ and tdTomato+/Lhx6+) if Lhx6 is required for cell survival.
Nkx2-1CreER pulse-chase experiments
Nkx2-1CreER/+;Ai9 female mice were time-mated to the same genotype male mice, and 4-OHT was intraperitoneally injected (2 mg) at E11.5, and embryos were collected at E18.5.
Sleep deprivation
Six-hour sleep-deprivation experiments were performed on Lhx6-GFP male mice as previously described12.
Tissue fixation
Embryos and mice younger than weaning age (P21) were fixed in 4% paraformaldehyde (PFA) between 8 and 12 h at 4 °C, incubated in 30% sucrose overnight at 4 °C, and snap-frozen in OCT compound for histology analysis. Whole embryos were used for fixation until E14.5, and from E14.5, brains were dissected out for fixation. Mice older than weaning age were anesthetized by intraperitoneal injection of avertin and perfused with cold 4% PFA. Brains were post fixed for 2 h at 4 °C with 4% PFA and processed as described above.
Cryosectioning
Frozen brains were sectioned at 25 μm with a cryostat (Leica CM3050S) along either the coronal or sagittal plane, and transferred to SuperfrostTM Plus slides.
In situ hybridization (ISH)
Chromogenic and fluorescent ISH was performed as previously described to stain for Lhx6 (BC065077), Gfra1 (AW060572), Gfra2 (BE994145), Ret (AW123296), Dlx1 (BC079609), Calb1 (AW489595), Calb2 (AI836013), Gal (BC044055), Penk (AI836252), Tac1 (BE954293), Npy (AI848386), Sst (BE984677), Th (BF449409), Gad1 (AW121495), Nkx2-1 (BC080868), Nkx2-2 (BG110), Shh (BC063087), Prox1 (BE982394), Six3 (BE953775), Lhx8 (BE448496), and Lef1 (BC038305)4,55. RNAscope with probe targeting Lhx6 was tested on E13.5 mice following the manufacturer’s protocol. Images were taken under the Keyence BZ-X800 fluorescence microscope or Zeiss LSM 700 microscope, and processed with ImageJ56, and pixel density was measured as previously described57.
Immunostaining
Immunostaining was performed with mouse-anti-Lhx6-antibody (1:200, sc-271433, Santa Cruz), rat-anti-RFP (1:500, ABIN334653, antibodies-online), rabbit-anti-Dlx1 (1:500, a gift from Jay Lee), guinea-pig-anti-Dlx1 (1:500, a gift from Jay Lee), rabbit-anti-Nkx2-1 (1:500, EP1584Y, Abcam), mouse-anti-Nkx2-2 (1:100, 74.5A5, DSHB), mouse-anti-NeuN (1:2000, MAB377), and rabbit-anti-cFos (1:1000, 226003, Synaptic Systems) as previously described57, except that M.O.M blocking reagent (MKB-2213) was used following manufacturer’s instruction when mouse primary antibodies were used. Alexa FluorTM 488, 594, 647 secondary antibodies were used in 1:500 dilutions. Sections were mounted with DAPI-Vectamount (Vectorlabs) and imaged under a Keyence BZ-X800 fluorescence microscope or Zeiss LSM 700 microscope. All cell counting was done with ImageJ. Cell counting was conducted in multiple brain areas across developmental ages using standard reference atlases for orientation4,58, using DAPI counterstaining or NeuN staining as a guideline. For identification of ID and TT, criteria described in our previous study were used4. Three sections (every second section to avoid counting the same cell, <E15.5 = 2 sections) were used per region, and 4–6 brains collected from between two and three individual litters were used. Cell counting was conducted blinded.
Bulk RNA-sequencing
Lhx6 pulse-chase sample sequencing
Lhx6CreER/+;Ai9 and Lhx6CreER/lox;Baxlox/lox;Ai9 P1 pups were treated with 4-OHT as described above and collected at P10. Between 4 and 6 pups from 2 different litters were pooled per sample without regard to sex, and papain-based enzymatic dissociation was performed on the dissected hypothalamus as previously described24. Dissociated cells were flow-sorted for tdTomato signal, and between 25,000 and 30,000 cells were collected directly into TRIzolTM LS reagent. RNA was extracted using Direct-zol RNA kits (Zymo Research) and RNA-Sequencing libraries were made using stranded Total RNA-Seq library prep. 2 libraries were made for Lhx6CreER/+;Ai9, and three libraries were made for Lhx6CreER/+;Lhx6lox/+;Baxlox/lox;Ai9. Libraries were sequenced with Illumina NextSeq500, paired-end read of 75 bp, 50 million reads per library. Illumina adapters of sequenced libraries were trimmed using Cutadapt (v1.18)/TrimGalore (v0.5.0)59 with default parameters, library qualities were assessed using FastQC (v0.11.7)/MultiQC60. Libraries were then aligned to mm10 using STAR (v2.54b)61 with –twopassMode Basic. RSEM (v1.3.0) was used for quantification62, with rsem-calculate-expression (–forward-prob 0.5). Expected counts value from RSEM was used to perform differential expression using edgeR (v3.24.3)63 using default parameters except for calcNormFactors (method = “TMM”).
Lhx6CreER/+;Ai9 or Lhx6CreER/lox;Baxlox/lox;Ai9 enriched genes (fold change > 2 consistent gene value across replicates), were used with EnrichR64. Lhx6lox/+;Baxlox/lox;Ai9 enriched genes were compared to the Mouse Cells and Tissues (MESA) dataset available ascot.cs.jhu.edu26, relying on robustness of expression (NAUC >20) and specificity, as many of the enriched genes detected in this analysis are not strongly expressed in the developing brain.
We reasoned that the genes showing enriched expression in Lhx6CreER/+;Ai9 relative to Lhx6CreER/lox;Baxlox/lox;Ai9 would be regulated by Lhx6 and/or Bax. Furthermore, since tdTomato expression is detected in blood vessels due to weak Lhx6 expression in endothelial neurons during development12, we wanted to enrich expression from Lhx6-expressing neurons of the hypothalamus. P8 Lhx6-GFP, in which GFP expression is absent in endothelial cells, was used to generate bulk RNA-Sequencing (bulk RNA-Seq) from the cortex and hypothalamus (method described below). Hypothalamus-enriched genes from P8 Lhx6-GFP bulk RNA-Seq data were used to enrich genes that are highly expressed in the hypothalamus Lhx6 neurons. After enrichment, the gene lists were compared to scRNA-Seq data from P8 Lhx6-GFP hypothalamus using the method described below, to further cross-check specificity of expression and to remove any possible contamination that may occur during flow sorting from bulk RNA-Seq. EnrichR was used to identify gene pathways, and pathways previously implicated in the regulation of neuronal survival were selected.
Lhx6-GFP bulk RNA-seq
To identify differences between cortical and hypothalamic Lhx6 populations, RNA-Sequencing was performed on E15.5, P0, and P8 Lhx6-GFP mice, by collecting 8–10 pups from two different litters per library. Libraries were sequenced with Illumina HiSeq 2500, and processed as described in the pipeline described above.
ATAC-sequencing
Cortex and hypothalamus of E15.5 and P0 Lhx6-GFP mice were collected, dissociated with papain-based enzymatic reaction, and GFP neurons were flow-sorted. Between 60,000 and 70,000 neurons were collected. Flow-sorted neurons were prepared for ATAC libraries as previously described65,66. Libraries were sequenced with Illumina NextSeq500, paired-end read of 75 bp, 50 million reads per library. Each sample was run in duplicate.
Illumina adapters of sequenced libraries were trimmed using Cutadapt (v1.18)/TrimGalore (v0.5.0) and library qualities were assessed using FastQC (v0.11.7)/MultiQC. Libraries were aligned to mm10 using Bowtie 2 (v2.25)67 using—very-sensitive parameter and Samtools (v1.9)68 was used to check the percentage of mitochondria DNA reads. Picard (v2.18) was used to remove PCR duplicates, and MACS2 (v2.1.2)69 was used to capture narrow peaks (open chromatin regions) with –shift 100, –extsize 200, –nolambda, –nomodel parameters. ENCODE blacklist regions of the genome were removed using Bedtools (v2.27) intersect function69,70,71. Bedtools intersect function was used to find matching peaks between replicates, in which the distance between peak ends was <10 base pairs. ChIPseeker (v1.18.0)72 was then used to identify regions that were within 3 kb of the transcription start site (TSS). Peaks between groups were compared as previously described65,66 to visualize changes in chromatin accessibility between different ages and brain regions using DiffBind (v.2.10.0)73 and edgeR using default parameters (FDR <0.05 and adjusted p value < 0.05). Differential peaks were compared to bulk RNA-Seq, and open chromatin peaks in promoter regions that correspond to altered gene expression from bulk RNA-Seq were identified65,66 to obtain a positive correlation between promoter accessibility and gene expression. Peaks and differential gene expression was then cross-matched to scRNA-Seq, to identify potential different regions within Lhx6 hypothalamic neurons that are demarcated by expression of specific transcription factors.
Single-cell RNA-sequencing
Time-mated E12.5, E15.5, and P8 Lhx6-GFP mice were collected, and dissection and dissociation were performed as described previously24. Between six and ten embryos/pups from two different litters were collected. Following dissociation, GFP+ neurons were flow-sorted using Aria IIu Sorter (BD). Between 20,000 and 25,000 neurons were flow-sorted for E12.5 and E15.5, 2000 neurons were flow-sorted for P8. Flow-sorted neurons were used for the 10× Genomics Chromium Single Cell System (10× Genomics, CA, USA) using V3.0 chemistry per manufacturer’s instruction. Three libraries were sequenced on Illumina NextSeq 500 with ~200 million reads per library. Sequenced files were processed through the CellRanger pipeline (v3.1.0, 10× Genomics) using mm10 genome.
Seurat V374 was used to perform downstream analysis following the standard pipeline described previously75, analyzing neurons that express a high Lhx6 transcript. Louvain algorithm was used to generate different clusters, and spatial information from individual clusters at E12.5 and E15.5 was identified by referring to our previous hypothalamus scRNA-Seq database HyDD24, as well as previous analysis of anatomical locations of transcription factors4. For P8 scRNA-Seq, region-specific transcription factors that are expressed were compared to E12.5 and E15.5 scRNA-Seq gene lists, as well as matching the identified gene lists to the Allen Brain Atlas ISH data58. Previously published scRNA-Seq from E13.5 MGE38 was processed as described above, and the key markers that label individual clusters were compared to E12.5 Lhx6-expressing hypothalamic neurons.
Lhx6+ neurons across multiple mutant groups (Foxd1Cre/+;Dlx1/2lox/lox, Nkx2-1CreER/CreER, Nkx2-2CreGFP/CreGFP) from24, were used to compare the expression level of key transcription factors that define sub-regions of hypothalamic Lhx6 expression domains.
Previously generated scRNA-Seq datasets from the preoptic region76, suprachiasmatic nucleus76,77, VMH78, and whole hypothalamus24,79,80,81, were analyzed as described above. GABAergic neurons (Slc32a1+) were first subsetted from the dataset, and the percentage of neurons expressing Pnoc, Penk, Calb1, Cck, Calb2, Gal, Tac1, Th, Npy, Trh, Sst was determined.
RNA velocity35 was used to understand the dynamic state of Lhx6 neuronal development, and RNA velocity was to identify (1) how Lhx6-expressing domains are established during development and (2) the origin of E15.5 Nkx2-2+ cluster. Kallisto and Bustools82,83 was used to obtain spliced and unspliced transcripts using --lamanno with GRCm38 mouse genome. Scanpy84 and scVelo85 was used to process the Kallisto output with default parameters, based on UMAP coordinates obtained from Seurat.
To identify regulatory transcription factors controlling gene expression in different Lhx6-expressing domains, SCENIC86,87 (python implemented pySCENIC (using –masks_dropouts)), was used to calculate regulatory transcription factors using default parameters with mm10 feather files on scRNA-Seq dataset using raw count matrix. This workflow involves three steps. This workflow involves three steps. First, we identify potential transcription factor targets in each cluster based on the co-expression of genes. Second, we perform transcription factor motif enrichment analysis and identify potential key regulatory transcription factors. Finally, we score the activity of these regulatory transcription factors based on the network of co-expressed genes.
Statistics
Two-way ANOVA was used for the Lhx6 pulse-chase experiments in Fig. 2 (genotype, brain region). Unpaired t test was used for all other cell counting studies. The Seurat “FindAllMarkers” function with “LR = logistic regression model” with default parameters was used for analyzing differential gene expression, using the number of total mRNAs and genes as a variable. All bar graphs show mean and standard error of the mean (SEM), with individual data points plotted.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
References
Lim, L., Mi, D., Llorca, A. & Marín, O. Development and functional diversification of cortical interneurons. Neuron 100, 294–313 (2018).
Huang, Z. J. & Paul, A. The diversity of GABAergic neurons and neural communication elements. Nat. Rev. Neurosci. 20, 563–572 (2019).
Erö, C., Gewaltig, M.-O., Keller, D. & Markram, H. A cell atlas for the mouse brain. Front. Neuroinform. 12, 28 (2018).
Shimogori, T. et al. A genomic atlas of mouse hypothalamic development. Nat. Neurosci. 13, 767–775 (2010).
Bedont, J. L., Newman, E. A. & Blackshaw, S. Patterning, specification, and differentiation in the developing hypothalamus. Wiley Interdiscip. Rev. Dev. Biol. 4, 445–468 (2015).
Colasante, G. et al. Arx is a direct target of Dlx2 and thereby contributes to the tangential migration of GABAergic interneurons. J. Neurosci. 28, 10674–10686 (2008).
Cobos, I. et al. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat. Neurosci. 8, 1059–1068 (2005).
Bulfone, A. et al. Spatially restricted expression of Dlx-1, Dlx-2 (Tes-1), Gbx-2, and Wnt- 3 in the embryonic day 12.5 mouse forebrain defines potential transverse and longitudinal segmental boundaries. J. Neurosci. 13, 3155–3172 (1993).
Bedont, J. L. et al. An LHX1-regulated transcriptional network controls sleep/wake coupling and thermal resistance of the central circadian clockworks. Curr. Biol. 27, 128–136 (2017).
Bedont, J. L. et al. Lhx1 controls terminal differentiation and circadian function of the suprachiasmatic nucleus. Cell Rep. 7, 609–622 (2014).
Hatori, M. et al. Lhx1 maintains synchrony among circadian oscillator neurons of the SCN. eLife 3, e03357 (2014).
Liu, K. et al. Lhx6-positive GABA-releasing neurons of the zona incerta promote sleep. Nature 548, 582–587 (2017).
Maroof, A. M., Brown, K., Shi, S. H., Studer, L. & Anderson, S. A. Prospective isolation of cortical interneuron precursors from mouse embryonic stem cells. J. Neurosci. 30, 4667–4675 (2010).
Kessaris, N., Magno, L., Rubin, A. N. & Oliveira, M. G. Genetic programs controlling cortical interneuron fate. Curr. Opin. Neurobiol. 26, 79–87 (2014).
Wang, Y. et al. Dlx5 and Dlx6 regulate the development of parvalbumin-expressing cortical interneurons. J. Neurosci. 30, 5334–5345 (2010).
Du, T., Xu, Q., Ocbina, P. J. & Anderson, S. A. NKX2.1 specifies cortical interneuron fate by activating Lhx6. Development 135, 1559–1567 (2008).
Fogarty, M. et al. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J. Neurosci. 27, 10935–10946 (2007).
Flandin, P. et al. Lhx6 and Lhx8 coordinately induce neuronal expression of Shh that controls the generation of interneuron progenitors. Neuron 70, 939–950 (2011).
Sandberg, M. et al. Transcriptional networks controlled by NKX2-1 in the development of forebrain GABAergic neurons. Neuron 91, 1260–1275 (2016).
Batista-Brito, R. et al. The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron 63, 466–481 (2009).
Zhao, Y. et al. Distinct molecular pathways for development of telencephalic interneuron subtypes revealed through analysis of Lhx6 mutants. J. Comp. Neurol. 510, 79–99 (2008).
Liodis, P. et al. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J. Neurosci. 27, 3078–3089 (2007).
Vogt, D. et al. Lhx6 directly regulates Arx and CXCR7 to determine cortical interneuron fate and laminar position. Neuron 82, 350–364 (2014).
Kim, D. W. et al. The cellular and molecular landscape of hypothalamic patterning and differentiation. bioRxiv https://doi.org/10.1101/657148 (2019).
Takeuchi, O. et al. Essential role of BAX,BAK in B cell homeostasis and prevention of autoimmune disease. Proc. Natl Acad. Sci. U.S.A. 102, 11272–11277 (2005).
Ling, J. P. et al. ASCOT identifies key regulators of neuronal subtype-specific splicing. Nat. Commun. 11, 137 (2020).
Denaxa, M. et al. Maturation-promoting activity of SATB1 in MGE-derived cortical interneurons. Cell Rep. 2, 1351–1362 (2012).
Flames, N. et al. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron 44, 251–261 (2004).
Li, H., Chou, S.-J., Hamasaki, T., Perez-Garcia, C. G. & O’Leary, D. D. M. Neuregulin repellent signaling via ErbB4 restricts GABAergic interneurons to migratory paths from ganglionic eminence to cortical destinations. Neural Dev. 7, 1–17 (2012).
Bartolini, G. et al. Neuregulin 3 mediates cortical plate invasion and laminar allocation of GABAergic interneurons. Cell Rep. 18, 1157–1170 (2017).
Mei, L. & Xiong, W.-C. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 9, 437–452 (2008).
Tasic, B. et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat. Neurosci. 19, 335–346 (2016).
Mickelsen, L. E. et al. Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nat. Neurosci. 22, 642–656 (2019).
Rossi, M. A. et al. Obesity remodels activity and transcriptional state of a lateral hypothalamic brake on feeding. Science 364, 1271–1274 (2019).
La Manno, G. et al. RNA velocity of single cells. Nature 560, 494–498 (2018).
Wonders, C. P. et al. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev. Biol. 314, 127–136 (2008).
Stenman, J. M., Wang, B. & Campbell, K. Tlx controls proliferation and patterning of lateral telencephalic progenitor domains. J. Neurosci. 23, 10568–10576 (2003).
Mayer, C. et al. Developmental diversification of cortical inhibitory interneurons. Nature 555, 457–462 (2018).
Anderson, S. A. et al. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron 19, 27–37 (1997).
Shimamura, K., Hartigan, D. J., Martinez, S., Puelles, L. & Rubenstein, J. L. Longitudinal organization of the anterior neural plate and neural tube. Development 121, 3923–3933 (1995).
Taniguchi, H. et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013 (2011).
Marín, O., Baker, J., Puelles, L. & Rubenstein, J. L. R. Patterning of the basal telencephalon and hypothalamus is essential for guidance of cortical projections. Development 129, 761–773 (2002).
Balderes, D. A., Magnuson, M. A. & Sussel, L. Nkx2.2:Cre knock-in mouse line: A novel tool for pancreas- and CNS-specific gene deletion. Genesis 51, 844–851 (2013).
Qiu, M. et al. Role of the Dlx homeobox genes in proximodistal patterning of the branchial arches: mutations of Dlx-1, Dlx-2, and Dlx-1 and -2 alter morphogenesis of proximal skeletal and soft tissue structures derived from the first and second arches. Dev. Biol. 185, 165–184 (1997).
Silbereis, J. C. et al. Olig1 function is required to repress dlx1/2 and interneuron production in Mammalian brain. Neuron 81, 574–587 (2014).
Newman, E. A., Wu, D., Taketo, M. M., Zhang, J. & Blackshaw, S. Canonical Wnt signaling regulates patterning, differentiation and nucleogenesis in mouse hypothalamus and prethalamus. Dev. Biol. 442, 236–248 (2018).
Salvatierra, J. et al. The LIM homeodomain factor Lhx2 is required for hypothalamic tanycyte specification and differentiation. J. Neurosci. 34, 16809–16820 (2014).
Pozas, E. & Ibáñez, C. F. GDNF and GFRalpha1 promote differentiation and tangential migration of cortical GABAergic neurons. Neuron 45, 701–713 (2005).
Abecassis, Z. A. et al. Npas1+-Nkx2.1+ neurons are an integral part of the cortico-pallido-cortical Loop. J. Neurosci. 40, 743–768 (2020).
Gong, S. et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925 (2003).
Denaxa, M. et al. Modulation of apoptosis controls inhibitory interneuron number in the cortex. Cell Rep. 22, 1710–1721 (2018).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Humphreys, B. D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97 (2010).
Watson, C. & Paxinos, G. Chemoarchitectonic Atlas of the Mouse Brain. (Academic Press, 2010).
Miranda-Angulo, A. L., Byerly, M. S., Mesa, J., Wang, H. & Blackshaw, S. Rax regulates hypothalamic tanycyte differentiation and barrier function in mice. J. Comp. Neurol. 522, 876–899 (2014).
Rueden, C. T. et al. ImageJ2: imageJ for the next generation of scientific image data. BMC Bioinform. 18, 529 (2017).
Kim, D. W., Glendining, K. A., Grattan, D. R. & Jasoni, C. L. Maternal obesity in the mouse compromises the blood-brain barrier in the arcuate nucleus of offspring. Endocrinology 157, 2229–2242 (2016).
Lein, E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 17, 10 (2011).
Ewels, P., Magnusson, M., Lundin, S. & Käller, M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32, 3047–3048 (2016).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 12, 323 (2011).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Kuleshov, M. V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97 (2016).
Wang, J. et al. ATAC-Seq analysis reveals a widespread decrease of chromatin accessibility in age-related macular degeneration. Nat. Commun. 9, 1364 (2018).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive mulitmodal analysis of chromatin architecture. Biophys. J. 106, 77a (2014).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Amemiya, H. M., Kundaje, A. & Boyle, A. P. The ENCODE blacklist: identification of problematic regions of the genome. Sci. Rep. 9, 9354 (2019).
Yu, G., Wang, L.-G. & He, Q.-Y. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31, 2382–2383 (2015).
Ross-Innes, C. S. et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481, 389–393 (2012).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 (2019). e21.
Bell, B. J. et al. A clock-driven neural network critical for arousal. bioRxiv. https://doi.org/10.1101/2020.03.12.989921 (2020).
Moffitt, J. R. et al. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 362, eaau5324 (2018).
Wen, S. ’ang et al. Spatiotemporal single-cell analysis of gene expression in the mouse suprachiasmatic nucleus. Nat. Neurosci. 23, 456–467 (2020).
Kim, D.-W. et al. Multimodal analysis of cell types in a hypothalamic node controlling social behavior. Cell 179, 713–728.e17 (2019).
Romanov, R. A. et al. Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat. Neurosci. 20, 176–188 (2017).
Chen, R., Wu, X., Jiang, L. & Zhang, Y. Single-cell RNA-seq reveals hypothalamic cell diversity. Cell Rep. 18, 3227–3241 (2017).
Campbell, J. N. et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci. 20, 484–496 (2017).
Melsted, P. et al. Modular and efficient pre-processing of single-cell RNA-seq. bioRxiv. https://doi.org/10.1101/673285.
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016).
Alexander Wolf, F., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Bergen, V., Lange, M., Peidli, S., Alexander Wolf, F. & Theis, F. J. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotechnol. 38,1408–1414 (2020).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Kim, D.W. et al. Gene regulatory networks controlling differentiation, survival, and diversification of hypothalamic Lhx6-expressing GABAergic neurons. Gene Expression Omnibus. Series GSE150687. (2020).
Acknowledgements
This work was supported by a grant from the NIH (R01DK108230) to S.B., the Maryland Stem Cell Research Fund (2019-MSCRFF-5124) to D.W.K., and the Japan Society for the Promotion of Science to T.S. We thank Transcriptomics and Deep Sequencing Core (Johns Hopkins) for sequencing of bulk RNA-Seq, bulk ATAC-Seq and scRNA-Seq libraries, and Ross Flow Cytometry Core (Johns Hopkins) for flow sorting of Lhx6-GFP cells, and Microscope facility (Johns Hopkins MICFAC, supported by the award number S10OD018118). We thank Marysia Placzek and Wendy Yap for comments on the paper.
Author information
Authors and Affiliations
Contributions
S.B. conceived the study. D.W.K., K.L. and S.B. designed experiments. D.W.K., K.L., Z.Q.W., S.Z., A.B., M.P.B., S.H.L., P.W.W. and T.S. performed experiments. D.W.K., K.L., Z.Q.W., S.Z., P.W.W., C.S. and T.S. analyzed data. B.L. and J.L.R. provided reagents. All authors contributed to writing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, D.W., Liu, K., Wang, Z.Q. et al. Gene regulatory networks controlling differentiation, survival, and diversification of hypothalamic Lhx6-expressing GABAergic neurons. Commun Biol 4, 95 (2021). https://doi.org/10.1038/s42003-020-01616-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-020-01616-7
This article is cited by
-
Dorsoventral Arrangement of Lateral Hypothalamus Populations in the Mouse Hypothalamus: a Prosomeric Genoarchitectonic Analysis
Molecular Neurobiology (2023)
-
Populational heterogeneity and partial migratory origin of the ventromedial hypothalamic nucleus: genoarchitectonic analysis in the mouse
Brain Structure and Function (2023)
-
Amyloid-beta and tau pathologies act synergistically to induce novel disease stage-specific microglia subtypes
Molecular Neurodegeneration (2022)
-
Ontogenetic rules for the molecular diversification of hypothalamic neurons
Nature Reviews Neuroscience (2022)
-
Evolutionarily conservative and non-conservative regulatory networks during primate interneuron development revealed by single-cell RNA and ATAC sequencing
Cell Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.