Abstract
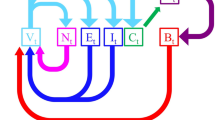
Pharmacologically isolated starburst amacrine cells (SACs) in perinatal rabbit retinas spontaneously generated semiperiodic calcium spikes and long-lasting after-hyperpolarizations (AHPs), mediated by calcium-activated, cyclic AMP–sensitive potassium currents. These AHPs, rather than a depletion of neurotransmitters (as was previously believed), produced the refractory period of spontaneous retinal waves and set the upper limit of the wave frequency. Each SAC received inputs from roughly 10–30 neighboring SACs during a wave. These inputs synchronized and reshaped the intrinsic bursts to produce network oscillations at a rhythm different from that of individual SACs. With maturation, the semiperiodic bursts in SACs disappeared, owing to reduced intrinsic excitability and increased network inhibition. Thus, retinal waves are generated by a transient and specific network of cell-autonomous oscillators synchronized by reciprocally excitatory connections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Wong, R.O. Retinal waves and visual system development. Annu. Rev. Neurosci. 22, 29–47 (1999).
Torborg, C.L. & Feller, M.B. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog. Neurobiol. 76, 213–235 (2005).
Stellwagen, D. & Shatz, C.J. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron 33, 357–367 (2002).
Torborg, C.L., Hansen, K.A. & Feller, M.B. High frequency, synchronized bursting drives eye-specific segregation of retinogeniculate projections. Nat. Neurosci. 8, 72–78 (2005).
McLaughlin, T., Torborg, C.L., Feller, M.B. & O'Leary, D.D. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron 40, 1147–1160 (2003).
Grubb, M.S., Rossi, F.M., Changeux, J.P. & Thompson, I.D. Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the beta 2 subunit of the nicotinic acetylcholine receptor. Neuron 40, 1161–1172 (2003).
Chandrasekaran, A.R., Plas, D.T., Gonzalez, E. & Crair, M.C. Evidence for an instructive role of retinal activity in retinotopic map refinement in the superior colliculus of the mouse. J. Neurosci. 25, 6929–6938 (2005).
Mrsic-Flogel, T.D. et al. Altered map of visual space in the superior colliculus of mice lacking early retinal waves. J. Neurosci. 25, 6921–6928 (2005).
Feller, M.B. Spontaneous correlated activity in developing neural circuits. Neuron 22, 653–656 (1999).
O'Donovan, M.J. The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr. Opin. Neurobiol. 9, 94–104 (1999).
Luthi, A. & McCormick, D.A. H-current: properties of a neuronal and network pacemaker. Neuron 21, 9–12 (1998).
Ramirez, J.M., Tryba, A.K. & Pena, F. Pacemaker neurons and neuronal networks: an integrative view. Curr. Opin. Neurobiol. 14, 665–674 (2004).
Buzsaki, G. & Draguhn, A. Neuronal oscillations in cortical networks. Science 304, 1926–1929 (2004).
Zheng, J.J., Lee, S. & Zhou, Z.J. A developmental switch in the excitability and function of the starburst network in the mammalian retina. Neuron 44, 851–864 (2004).
Syed, M.M., Lee, S., Zheng, J. & Zhou, Z.J. Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina. J. Physiol. (Lond.) 560, 533–549 (2004).
Bansal, A. et al. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J. Neurosci. 20, 7672–7681 (2000).
Feller, M.B., Wellis, D.P., Stellwagen, D., Werblin, F.S. & Shatz, C.J. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science 272, 1182–1187 (1996).
Zhou, Z.J. & Zhao, D. Coordinated transitions in neurotransmitter systems for the initiation and propagation of spontaneous retinal waves. J. Neurosci. 20, 6570–6577 (2000).
Wong, W.T., Myhr, K.L., Miller, E.D. & Wong, R.O. Developmental changes in the neurotransmitter regulation of correlated spontaneous retinal activity. J. Neurosci. 20, 351–360 (2000).
Zhou, Z.J. The function of the cholinergic system in the developing mammalian retina. Prog. Brain Res. 131, 599–613 (2001).
Zhou, Z.J. Direct participation of starburst amacrine cells in spontaneous rhythmic activities in the developing mammalian retina. J. Neurosci. 18, 4155–4165 (1998).
Ames, A. & Nesbett, F.B. In vitro retina as an experimental model of the central nervous system. J. Neurochem. 37, 867–877 (1981).
Stellwagen, D., Shatz, C.J. & Feller, M.B. Dynamics of retinal waves are controlled by cyclic AMP. Neuron 24, 673–685 (1999).
Faber, E.S. & Sah, P. Calcium-activated potassium channels: multiple contributions to neuronal function. Neuroscientist 9, 181–194 (2003).
Lancaster, B. & Adams, P.R. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J. Neurophysiol. 55, 1268–1282 (1986).
Lancaster, B. & Nicoll, R.A. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J. Physiol. (Lond.) 389, 187–203 (1987).
Sah, P. & Clements, J.D. Photolytic manipulation of [Ca2+]i reveals slow kinetics of potassium channels underlying the after hyperpolarization in hippocampal pyramidal neurons. J. Neurosci. 19, 3657–3664 (1999).
Sah, P. Ca(2+)-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 19, 150–154 (1996).
Sah, P. & Faber, E.S. Channels underlying neuronal calcium-activated potassium currents. Prog. Neurobiol. 66, 345–353 (2002).
Vogalis, F., Storm, J.F. & Lancaster, B. SK channels and the varieties of slow after-hyperpolarizations in neurons. Eur. J. Neurosci. 18, 3155–3166 (2003).
Shah, M.M., Miscony, Z., Javadzadeh-Tabatabaie, M., Ganellin, C.R. & Haylett, D.G. Clotrimazole analogues: effective blockers of the slow afterhyperpolarization in cultured rat hippocampal pyramidal neurones. Br. J. Pharmacol. 132, 889–898 (2001).
Ozaita, A. et al. A unique role for Kv3 voltage-gated potassium channels in starburst amacrine cell signaling in mouse retina. J. Neurosci. 24, 7335–7343 (2004).
Sipila, S.T., Huttu, K., Soltesz, I., Voipio, J. & Kaila, K. Depolarizing GABA acts on intrinsically bursting pyramidal neurons to drive giant depolarizing potentials in the immature hippocampus. J. Neurosci. 25, 5280–5289 (2005).
Tauchi, M. & Masland, R.H. The shape and arrangement of the cholinergic neurons in the rabbit retina. Proc. R. Soc. Lond. B 223, 101–119 (1984).
Vaney, D.I. 'Coronate' amacrine cells in the rabbit retina have the 'starburst' dendritic morphology. Proc. R. Soc. Lond. B 220, 501–508 (1984).
Famiglietti, E.V. Starburst amacrine cells: morphological constancy and systematic variation in the anisotropic field of rabbit retinal neurons. J. Neurosci. 5, 562–577 (1985).
Feller, M.B., Butts, D.A., Aaron, H.L., Rokhsar, D.S. & Shatz, J.C. Dynamic processes shape spatiotemporal properties of retinal waves. Neuron 19, 293–306 (1997).
Jackson, A.C., Yao, G.L. & Bean, B.P. Mechanism of spontaneous firing in dorsomedial suprachiasmatic nucleus neurons. J. Neurosci. 24, 7985–7998 (2004).
McCormick, D.A. & Huguenard, J.R. A model of the electrophysiological properties of thalamocortical relay neurons. J. Neurophysiol. 68, 1384–1400 (1992).
Huguenard, J.R. & McCormick, D.A. Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. J. Neurophysiol. 68, 1373–1383 (1992).
Schwindt, P.C., Spain, W.J. & Crill, W.E. Effects of intracellular calcium chelation on voltage-dependent and calcium-dependent currents in cat neocortical neurons. Neuroscience 47, 571–578 (1992).
Zhang, L. et al. Potentiation of a slow Ca(2+)-dependent K+ current by intracellular Ca2+ chelators in hippocampal CA1 neurons of rat brain slices. J. Neurophysiol. 74, 2225–2241 (1995).
Velumian, A.A. & Carlen, P.L. Differential control of three after-hyperpolarizations in rat hippocampal neurones by intracellular calcium buffering. J. Physiol. (Lond.) 517, 201–216 (1999).
Petit-Jacques, J., Volgyi, B., Rudy, B. & Bloomfield, S.A. Spontaneous oscillatory activity of starburst amacrine cells in the mouse retina. J. Neurophysiol. 94, 1770–1780 (2005).
Acknowledgements
We thank Q. Yang and T. Mon for help with some imaging experiments and A. Hayar for discussions on cross-correlation analysis. This study was supported by US National Institutes of Health grant R01EY10894 (to Z.J.Z.), unrestricted funds from Research to Prevent Blindness Inc. and from the Pat and Willard Walker Eye Research Center, and by the University of Arkansas for Medical Sciences Tobacco Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Cross-correlation analysis among neighboring starburst amacrine cells (SACs) under Ca2+ imaging. (PDF 96 kb)
Supplementary Fig. 2
Excitability of SACs during the slow AHP. (PDF 445 kb)
Rights and permissions
About this article
Cite this article
Zheng, J., Lee, S. & Zhou, Z. A transient network of intrinsically bursting starburst cells underlies the generation of retinal waves. Nat Neurosci 9, 363–371 (2006). https://doi.org/10.1038/nn1644
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1644
This article is cited by
-
Modeling cholinergic retinal waves: starburst amacrine cells shape wave generation, propagation, and direction bias
Scientific Reports (2023)
-
On the potential role of lateral connectivity in retinal anticipation
The Journal of Mathematical Neuroscience (2021)
-
A biophysical model explains the spontaneous bursting behavior in the developing retina
Scientific Reports (2019)
-
Nanowire arrays restore vision in blind mice
Nature Communications (2018)
-
Cortical travelling waves: mechanisms and computational principles
Nature Reviews Neuroscience (2018)