Abstract
Eph receptor tyrosine kinases and their ephrin ligands are involved in crucial aspects of nervous system circuit assembly during development1,2, but their functional roles in the mature nervous system are poorly understood. We investigated their role in pain processing, using a combination of immunohistochemical, behavioral, biochemical and primary cell culture techniques. Here we report an in vivo role for EphB–ephrinB interactions as modulators of synaptic efficacy in the spinal cord, contributing to sensory abnormalities in persistent pain states.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wilkinson, D.G. Nat. Rev. Neurosci. 2, 155–164 (2001).
Flanagan, J.G. & Vanderhaeghen, P. Annu. Rev. Neurosci. 21, 309–345 (1998).
Dalva, M.B. et al. Cell 103, 945–956 (2000).
Takasu, M.A., Dalva, M.B., Zigmond, R.E. & Greenberg, M.E. Science 295, 491–495 (2002).
Grunwald, I.C. et al. Neuron 32, 1027–1040 (2001).
Henderson, J.T. et al. Neuron 32, 1041–1056 (2001).
Ghosh, A. Science 295, 449–451 (2002).
McMahon, S.B., Lewin, G.R. & Wall, P.D. Curr. Opin. Neurobiol. 3, 602–610 (1993).
Stein, E. et al. Genes Devel. 12, 667–678 (1998).
Conover, J.C. et al. Nat. Neurosci. 3, 1091–1097 (2000).
Murai, K.K. & Pasquale, E.B. Neuron 33, 159–162 (2002).
Hargreaves, K., Dubner, R., Brown, F., Flores, C. & Joris, J. Pain 32, 77–88 (1988).
Honore, P., Buritova, J. & Besson, J.M. Eur. J. Pharmacol. 272, 249–259 (1995).
Acknowledgements
We thank T. Cowen, M. Geymonat, M. Malcangio, C.Thravivoulou, S. Pezet and J.N. Wood for their help. Supported by Wellcome Trust PG 060455.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1.
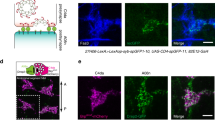
Ephrin-B2 is expressed presynaptically (on sensory afferents) and EphB1 postsynaptically in spinal cord DH. Ephrin-B2 was the most widely expressed ephrin in lumbar DRG, present in ~44.8% (± 1.12 s.e.m) of neurons, virtually all of small diameter (<35 μm). Ephrin-B2 positive neurons were negative for RT97 (a marker for large and intermediate neurons). All VR1-expressing neurons and 79 ± 5.6% of P2X3 immunoreactive neurons were positive for ephrinB2. P2X3 and VR1 are markers for nociceptive neurons. Some small neurons showed staining for ephrin-B1, but none for ephrin-B3 (not shown). Terminals that had ephrin-B2, but not ephrin-B1, were visible in DH laminae I and II. Many DH neurons in laminae I-III showed EphB1 receptor immunoreactivity. Inset, motor neurons. Scale bars, 100 μm. (PDF 940 kb)
Supplementary Fig. 2.
EphrinB2-Fc i.t. treatment does not affect withdrawal latencies following mechanical non-noxious stimulation of the hindpaw. Repeated measurements from rats treated i.t. with human Fc fragment (control) or ephrinB2-Fc (at 2 or 10 μg/rat). (PDF 649 kb)
Supplementary Fig. 3.
Intrathecally delivered EphB1-Fc significantly reduces pain-related behavior in Phase II of the formalin response. Charts represent time course for pain behaviors induced by 1.5% formalin after i.t injection of human Fc (control) or EphB1-Fc. (a) Time course for lifting. EphB1-Fc i.t. injection significantly (P < 0.001) reduced the amount of time the animals spent with the formalin injected paw lifted off the floor. (b) Pain scores (calculated on the basis of the pain related behaviors of favouring, lifting and licking/biting). EphB1 i.t. injection significantly (P < 0.001) reduces pain scores in Phase II of the formalin response. (PDF 999 kb)
Supplementary Fig. 4.
ATP stimulation alters the intensity and pattern of ephrin-B2 staining in DRG neurons in culture. Confocal images of cultured rat DRG neurons, untreated or stimulated with 100 µM ATP and labelled with ephrin-B2 antibodies. For illustrative purposes images are pseudocolored to illustrate intensity of staining (lighter colour indicates higher intensity). Ephrin-B2 immunoreactivity was observed in neurons on soma, along neurites and at the level of growth cones. Note aggregates of staining in the ATP-treated neuron on the growth cone presented in the boxed area. Scale bar, 50 μm. ATP-treated neurons were significantly brighter and had a larger number of separate peaks of high intensity staining, presumably indicative of clustering, as compared to controls. Densitometric analysis was performed with LaserPict software on single optical sections. Grey value measurements for ATP-treated neurons were expressed as percentages of control. Clustering of ephrin-B2 staining was measured by counting the number of separate peaks above average grey value along randomly selected segments of neurite (500 pixels/field). A total of 3904 μm of neurites were sampled. Cells which were P2X3-immunopositive (and therefore ATP-responsive) showed higher levels of ephrin-B2 immunoreactivity in image analysis as compared to P2X3-immunopositive cells in untreated cultures (143 ±11% vs control; P < 0.05, unpaired t-test). The number of discrete peaks of ephrin-B2 immunofluorescence/unit length was significantly higher in ATP-treated neurons (119 ± 3.5 % vs. control, P < 0.01, unpaired t-test), indicating the presence of a higher number of ephrin-B2 clusters. (PDF 42 kb)
Rights and permissions
About this article
Cite this article
Battaglia, A., Sehayek, K., Grist, J. et al. EphB receptors and ephrin-B ligands regulate spinal sensory connectivity and modulate pain processing. Nat Neurosci 6, 339–340 (2003). https://doi.org/10.1038/nn1034
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1034
This article is cited by
-
EphrinB/EphB forward signaling in Müller cells causes apoptosis of retinal ganglion cells by increasing tumor necrosis factor alpha production in rat experimental glaucomatous model
Acta Neuropathologica Communications (2018)
-
The transition from acute to chronic pain: understanding how different biological systems interact
Canadian Journal of Anesthesia/Journal canadien d'anesthésie (2014)
-
Temporal Control of Gene Deletion in Sensory Ganglia Using a Tamoxifen-Inducible Advillin-CreERT2 Recombinase Mouse
Molecular Pain (2011)
-
Long-Term Potentiation in Spinal Nociceptive Pathways as a Novel Target for Pain Therapy
Molecular Pain (2011)
-
Nociceptor-Expressed Ephrin-B2 Regulates Inflammatory and Neuropathic Pain
Molecular Pain (2010)