Abstract
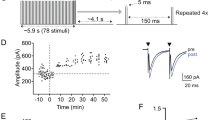
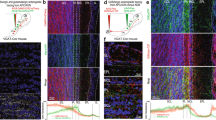
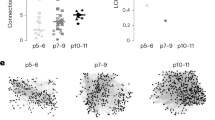
Synapse elimination and strengthening are central mechanisms for the developmental organization of excitatory neuronal networks. Little is known, however, about whether these processes are also involved in establishing precise inhibitory circuits. We examined the development of functional connectivity before hearing onset in rats in the tonotopically organized, glycinergic pathway from the medial nucleus of the trapezoid body (MNTB) to the lateral superior olive (LSO), which is part of the mammalian sound localization system. We found that LSO neurons became functionally disconnected from ∼75% of their initial inputs, resulting in a two-fold sharpening of functional topography. This was accompanied by a 12-fold increase in the synaptic conductance generated by maintained individual inputs. Functional elimination of MNTB–LSO synapses was restricted to the period when these glycinergic/GABAergic synapses are excitatory. These results provide new insights into the mechanisms by which precisely organized inhibitory circuits are established during development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Purves, D. & Lichtman, J.W. Principles of Neural Development (Sinauer Associates Inc., Sunderland, 1985).
Lichtman, J.W. & Colman, H. Synapse elimination and indelible memory. Neuron 25, 269–278 (2000).
Goodman, C.S. & Shatz, C.J. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell 72 (Suppl.), 77–98 (1993).
Jackson, H. & Parks, T.N. Functional synapse elimination in the developing avian cochlear nucleus with simultaneous reduction in cochlear nerve axon branching. J. Neurosci. 2, 1736–1743 (1982).
Chen, C. & Regehr, W.G. Developmental remodeling of the retinogeniculate synapse. Neuron 28, 955–966 (2000).
Stellwagen, D. & Shatz, C.J. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron 33, 357–367 (2002).
Hubel, D.H., Wiesel, T.N. & LeVay, S. Plasticity of ocular dominance columns in monkey striate cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 278, 377–409 (1977).
Kilman, V., van Rossum, M.C. & Turrigiano, G.G. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABAA receptors clustered at neocortical synapses. J. Neurosci. 22, 1328–1337 (2002).
Rico, B., Xu, B. & Reichardt, L.F. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat. Neurosci. 5, 225–233 (2002).
Hendry, S.H. & Jones, E.G. Reduction in number of immunostained GABAergic neurons in deprived-eye dominance columns of monkey area 17. Nature 320, 750–753 (1986).
Hensch, T.K. et al. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282, 1504–1508 (1998).
Huang, Z.J. et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 739–755 (1999).
Rozas, C. et al. Developmental inhibitory gate controls the relay of activity to the superficial layers of the visual cortex. J. Neurosci. 21, 6791–6801 (2001).
Knudsen, E.I. Instructed learning in the auditory localization pathway of the barn owl. Nature 417, 322–328 (2002).
Kapfer, C., Seidl, A.H., Schweizer, H. & Grothe, B. Experience-dependent refinement of inhibitory inputs to auditory coincidence-detector neurons. Nat. Neurosci. 5, 247–253 (2002).
Sanes, D.H. & Friauf, E. Development and influence of inhibition in the lateral superior olivary nucleus. Hear. Res. 147, 46–58 (2000).
Rietzel, H.J. & Friauf, E. Neuron types in the rat lateral superior olive and developmental changes in the complexity of their dendritic arbors. J. Comp. Neurol. 390, 20–40 (1998).
Sanes, D.H. & Siverls, V. Development and specificity of inhibitory terminal arborizations in the central nervous system. J. Neurobiol. 22, 837–854 (1991).
Kotak, V.C., Korada, S., Schwartz, I.R. & Sanes, D.H. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J. Neurosci. 18, 4646–4655 (1998).
Boudreau, J.C. & Tsuchitani, C. Binaural interaction in the cat superior olive S segment. J. Neurophysiol. 31, 442–454 (1968).
Irvine, D.R.F. The mammalian auditory pathway: neurophysiology. in Springer Handbook of Auditory Research Vol. 2 (eds. Fay, R.R. & Popper, A.A.) 153–231 (Springer-Verlag, 1992).
Helfert, R.H. & Schwartz, I.R. Morphological evidence for the existence of multiple neuronal classes in the cat lateral superior olivary nucleus. J. Comp. Neurol. 244, 533–549 (1986).
Kandler, K., Givens, R.S. & Katz, L.C. in Imaging Neurons (eds. Yuste, R., Lanni, F. & Konnerth, A.) 271–279 (Cold Spring Harbor Laboratory Press, 1998).
Kullmann, P.H., Ene, F.A. & Kandler, K. Glycinergic and GABAergic calcium responses in the developing lateral superior olive. Eur. J. Neurosci. 15, 1093–1104 (2002).
Lohmann, C., Ilic, V. & Friauf, E. Development of a topographically organized auditory network in slice culture is calcium dependent. J. Neurobiol. 34, 97–112 (1998).
Friauf, E. Tonotopic order in the adult and developing auditory system of the rat as shown by c-fos immunocytochemistry. Eur. J. Neurosci. 4, 798–812 (1992).
von Gersdorff, H. & Borst, J.G. Short-term plasticity at the calyx of Held. Nat. Rev. Neurosci. 3, 53–64 (2002).
Sanes, D.H. The development of synaptic function and integration in the central auditory system. J. Neurosci. 13, 2627–2637 (1993).
Kandler, K. & Ecki, F. Development of electrical membrane properties and discharge characteristics of superior olivary complex neurons in fetal and postnatal rats. Eur. J. Neurosci. 7, 1773–1790 (1995).
Stevens, C.F. & Wang, Y. Changes in reliability of synaptic function as a mechanism for plasticity. Nature 371, 704–707 (1994).
Jonas, P., Bischofberger, J. & Sandkuhler, J. Co-release of two fast neurotransmitters at a central synapse. Science 281, 419–424 (1998).
Blatchley, B.J., Cooper, W.A. & Coleman, J.R. Development of auditory brainstem response to tone pip stimuli in the rat. Brain Res. 429, 75–84 (1987).
Colman, H., Nabekura, J. & Lichtman, J.W. Alterations in synaptic strength preceding axon withdrawal. Science 275, 356–361 (1997).
Antonini, A. & Stryker, M.P. Rapid remodeling of axonal arbors in the visual cortex. Science 260, 1819–1821 (1993).
Kandler, K. & Friauf, E. Development of glycinergic and glutamatergic synaptic transmission in the auditory brainstem of perinatal rats. J. Neurosci. 15, 6890–6904 (1995).
Ehrlich, I., Lohrke, S. & Friauf, E. Shift from depolarizing to hyperpolarizing glycine action in rat auditory neurones is due to age-dependent Cl− regulation. J. Physiol. 520, 121–137 (1999).
Ben Ari, Y. Developing networks play a similar melody. Trends Neurosci. 24, 353–360 (2001).
Kotak, V.C. & Sanes, D.H. Long-lasting inhibitory synaptic depression is age- and calcium- dependent. J. Neurosci. 20, 5820–5826 (2000).
Aizenman, C.D., Manis, P.B. & Linden, D.J. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron 21, 827–835 (1998).
Katz, L.C. & Shatz, C.J. Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138 (1996).
Kotak, V.C. & Sanes, D.H. Developmental influence of glycinergic transmission: regulation of NMDA receptor–mediated EPSPs. J. Neurosci. 16, 1836–1843 (1996).
Kirsch, J. & Betz, H. Glycine-receptor activation is required for receptor clustering in spinal neurons. Nature 392, 717–720 (1998).
Sanes, D.H. & Takacs, C. Activity-dependent refinement of inhibitory connections. Eur. J. Neurosci. 5, 570–574 (1993).
Gubellini, P., Ben Ari, Y. & Gaiarsa, J.L. Activity- and age-dependent GABAergic synaptic plasticity in the developing rat hippocampus. Eur. J. Neurosci. 14, 1937–1946 (2001).
Takahashi, T., Momiyama, A., Hirai, K., Hishinuma, F. & Akagi, H. Functional correlation of fetal and adult forms of glycine receptors with developmental changes in inhibitory synaptic receptor channels. Neuron 9, 1155–1161 (1992).
Bormann, J., Hamill, O.P. & Sakmann, B. Mechanism of anion permeation through channels gated by glycine and τ-aminobutyric acid in mouse cultured spinal neurones. J. Physiol. (Lond.) 385, 243–286 (1987).
Grantyn, R., Kraszewski, K., Melnick, I., Taschenberger, H. & Warton, S.S. In vitro development of vertebrate central synapses. Perspect. Dev. Neurobiol. 2, 387–397 (1995).
Davis, G.W. & Goodman, C.S. Synapse-specific control of synaptic efficacy at the terminals of a single neuron. Nature 392, 82–86 (1998).
Callaway, E.M. & Katz, L.C. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc. Natl. Acad. Sci. USA 90, 7661–7665 (1993).
Kaufman, L. & Rousseeuw, P.J. Finding Groups in Data: an Introduction to Cluster Analysis (Wiley, New York, 1990).
Acknowledgements
We thank N.K. Baba, E. Frank, D. Gillespie, E. Rubel and D. Simons for discussions and comments on the manuscript, I. Ehrlich for determining MNTB boundaries and N. Allman for technical support. We are also grateful to R. Givens for php-glutamate and to B. Schmidt for BC204 GABA. This work was supported by the National Institute on Deafness and Other Communicative Disorders (DC04199), the Alfred P. Sloan foundation, a Presidential Early Career Award (K.K.) and the Center for Neural Basis of Cognition (G.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig 1.
MNTB-elicited calcium responses in the immature superior olivary complex. Areas of calcium responses are outlined (different colors represent different stimulation trials) and overlaid on corresponding bright-field images. Calcium responses occur consistently in the LSO and only on rare occasions also in a few other nuclei such as the medial superior olive (MSO) and superior paraolivary nucleus (SPN). The absence of consistent responses in areas outside the LSO indicates that calcium responses in the immature LSO are elicited by monosynaptic MNTB-LSO connections rather than by polysynaptic pathways between the MNTB and LSO. Slices were prepared from P2 rats and bulk-labeled with Fura-2 AM. Changes in intracellular calcium concentrations were measured using 340/380 nm ratio-imaging on an inverted microscope (Nikon Eclipse TE200) with a 10x objective (NA: 0.5). For details of staining and imaging procedures, see ref. 24. The MNTB was stimulated electrically with bipolar stimulation electrodes (two pulses at 30 μA in A and 40 μA in B, 0.1 ms duration, separated by 20 ms). To increase spike activity and increase the detection of calcium responses, 10 mM TEA was included in the perfusion medium. Ionotropic glutamate receptors were blocked by CNQX (20 μM) and D,L- APV (100 μM) to isolate glycinergic/GABAergic calcium responses. Stimulus-induced calcium responses were detected by subtracting the ratio-image immediately taken before electrical stimulation from the first ratio-image taken after MNTB-stimulation. The resulting δratio-image was smoothed by a 3 x 3 Gaussian filter (Adobe Photoshop), thresholded, and overlaid onto the corresponding bright-field image. To map the entire superior olivary complex, 4-6 areas in the superior olivary complex were sequentially imaged and assembled using the corresponding bright-field images for orientation. At each position, responses to two stimulus trials were measured and all responses were overlaid over the composite image. (JPG 72 kb)
Supplementary Fig 2.
Absence of intra-nuclear synaptic connections between LSO neurons in neonatal rats as revealed by focal uncaging of glutamate. Locations of uncaging sites are indicated by circles and are overlaid on a brightfield video picture taken during the mapping experiments. LSO boundaries are outlined in black. Uncaging sites, which failed to elicit currents in the recorded LSO neuron, are marked in yellow; uncaging sites, which elicited currents, are marked in green. In all 6 LSO neurons tested, responses could only be elicited from 4-5 stimulation sites located in close vicinity to LSO cell bodies (location of cell bodies indicated by the tip of the recording electrode). Successful stimulation sites form elongated response areas (4.3 ± 0.2 locations, n = 6). The long axis of response areas matched the orientation of the bipolar dendrites of the recorded neurons as observed at 40x magnification during seal formation. Notably, responses were never elicited from medio-lateral ("tonotopically") distant stimulation sites. In 3 neurons (right columns), response areas were remapped after application of 1 μM TTX to block spike-elicited synaptic transmission. In two neurons, input patterns were completely unaffected by TTX, and in one neuron only one single input site could not be reproduced in TTX (lower row). This TTX-insensitivity indicates that responses resulted from direct stimulation of LSO dendrites rather then the activation of local intra-nuclear connections. Slices were prepared from P3-P4 animals and recordings were performed in voltage-clamp using a K-gluconate-based pipette solution (see Methods). (JPG 104 kb)
Rights and permissions
About this article
Cite this article
Kim, G., Kandler, K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci 6, 282–290 (2003). https://doi.org/10.1038/nn1015
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1015
This article is cited by
-
Corticostriatal Neurons in the Anterior Auditory Field Regulate Frequency Discrimination Behavior
Neuroscience Bulletin (2023)
-
Long-term microglia depletion impairs synapse elimination and auditory brainstem function
Scientific Reports (2022)
-
Long-term potentiation of glycinergic synapses by semi-natural stimulation patterns during tonotopic map refinement
Scientific Reports (2020)
-
Purinergic Modulation of Activity in the Developing Auditory Pathway
Neuroscience Bulletin (2020)
-
Assembly and maintenance of GABAergic and Glycinergic circuits in the mammalian nervous system
Neural Development (2018)