Abstract
The identity of cortical circuits mediating nociception and pain is largely unclear. The cingulate cortex is consistently activated during pain, but the functional specificity of cingulate divisions, the roles at distinct temporal phases of central plasticity and the underlying circuitry are unknown. Here we show in mice that the midcingulate division of the cingulate cortex (MCC) does not mediate acute pain sensation and pain affect, but gates sensory hypersensitivity by acting in a wide cortical and subcortical network. Within this complex network, we identified an afferent MCC–posterior insula pathway that can induce and maintain nociceptive hypersensitivity in the absence of conditioned peripheral noxious drive. This facilitation of nociception is brought about by recruitment of descending serotonergic facilitatory projections to the spinal cord. These results have implications for our understanding of neuronal mechanisms facilitating the transition from acute to long-lasting pain.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Vogt, B.A., Berger, G.R. & Derbyshire, S.W.G. Structural and functional dichotomy of human midcingulate cortex. Eur. J. Neurosci. 18, 3134–3144 (2003).
Vogt, B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 6, 533–544 (2005).
Shackman, A.J. et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 12, 154–167 (2011).
Wager, T.D. et al. An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 368, 1388–1397 (2013).
Xu, H. et al. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J. Neurosci. 28, 7445–7453 (2008).
Apps, M.A.J., Lockwood, P.L. & Balsters, J.H. The role of the midcingulate cortex in monitoring others' decisions. Front. Neurosci. 7, 251 (2013).
Vogt, B.A. & Paxinos, G. Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Struct. Funct. 219, 185–192 (2014).
Gao, Y.J., Ren, W.H., Zhang, Y.Q. & Zhao, Z.Q. Contributions of the anterior cingulate cortex and amygdala to pain- and fear-conditioned place avoidance in rats. Pain 110, 343–353 (2004).
Johansen, J.P. & Fields, H.L. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat. Neurosci. 7, 398–403 (2004).
Qu, C. et al. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain 152, 1641–1648 (2011).
Wei, F. et al. Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron 36, 713–726 (2002).
Li, X.Y. et al. Alleviating neuropathic pain hypersensitivity by inhibiting PKMζ in the anterior cingulate cortex. Science 330, 1400–1404 (2010).
Koga, K. et al. In vivo whole-cell patch-clamp recording of sensory synaptic responses of cingulate pyramidal neurons to noxious mechanical stimuli in adult mice. Mol. Pain 6, 62 (2010).
Kang, S.J. et al. Bidirectional modulation of hyperalgesia via the specific control of excitatory and inhibitory neuronal activity in the ACC. Mol. Brain 8, 81 (2015).
Ren, L.Y. et al. Distinct roles of the anterior cingulate cortex in spinal and supraspinal bee venom-induced pain behaviors. Neuroscience 153, 268–278 (2008).
Gu, L. et al. Pain inhibition by optogenetic activation of specific anterior cingulate cortical neurons. PLoS One 10, e0117746 (2015).
Schweinhardt, P. & Bushnell, M.C. Pain imaging in health and disease—how far have we come? J. Clin. Invest. 120, 3788–3797 (2010).
Hu, L. et al. The primary somatosensory cortex and the insula contribute differently to the processing of transient and sustained nociceptive and non-nociceptive somatosensory inputs. Hum. Brain Mapp. 36, 4346–4360 (2015).
Tye, K.M. & Deisseroth, K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat. Rev. Neurosci. 13, 251–266 (2012).
Luo, C. et al. Presynaptically localized cyclic GMP-dependent protein kinase 1 is a key determinant of spinal synaptic potentiation and pain hypersensitivity. PLoS Biol. 10, e1001283 (2012).
Meyer, R.A., Ringkamp, M., Campbell, J.N. & Raja, S.N. Peripheral mechanism of cutaneous nociception. in Wall and Melzack's Textbook of Pain (eds. McMahon, S.B. & Koltzenburg, M.) 3–34 (Elsevier Churchill Livingstone, 2006).
Mattis, J. et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods 9, 159–172 (2011).
Mahn, M., Prigge, M., Ron, S., Levy, R. & Yizhar, O. Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat. Neurosci. 19, 554–556 (2016).
Kayser, V. et al. Mechanical, thermal and formalin-induced nociception is differentially altered in 5-HT1A−/−, 5-HT1B−/−, 5-HT2A−/−, 5-HT3A−/− and 5-HTT−/− knock-out male mice. Pain 130, 235–248 (2007).
Frot, M., Mauguière, F., Magnin, M. & Garcia-Larrea, L. Parallel processing of nociceptive A-delta inputs in SII and midcingulate cortex in humans. J. Neurosci. 28, 944–952 (2008).
Apkarian, A.V., Baliki, M.N. & Geha, P.Y. Towards a theory of chronic pain. Prog. Neurobiol. 87, 81–97 (2009).
Chen, T. et al. Postsynaptic potentiation of corticospinal projecting neurons in the anterior cingulate cortex after nerve injury. Mol. Pain 10, 33 (2014).
Calejesan, A.A., Kim, S.J. & Zhuo, M. Descending facilitatory modulation of a behavioral nociceptive response by stimulation in the adult rat anterior cingulate cortex. Eur. J. Pain 4, 83–96 (2000).
Johansen, J.P., Fields, H.L. & Manning, B.H. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc. Natl. Acad. Sci. USA 98, 8077–8082 (2001).
Tang, J. et al. Pavlovian fear memory induced by activation in the anterior cingulate cortex. Mol. Pain 1, 6 (2005).
Singer, T. et al. Empathy for pain involves the affective but not sensory components of pain. Science 303, 1157–1162 (2004).
Kuner, R. & Flor, H. Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 18, 20–30 (2016).
Sheth, S.A. et al. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature 488, 218–221 (2012).
Zhang, Z. et al. Role of Prelimbic GABAergic Circuits in Sensory and Emotional Aspects of Neuropathic Pain. Cell Rep. 12, 752–759 (2015).
Lee, M. et al. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J. Neurosci. 35, 5247–5259 (2015).
Craig, A.D. How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70 (2009).
Segerdahl, A.R., Mezue, M., Okell, T.W., Farrar, J.T. & Tracey, I. The dorsal posterior insula subserves a fundamental role in human pain. Nat. Neurosci. 18, 499–500 (2015).
Davis, K.D., Bushnell, M.C., Iannetti, G.D., St Lawrence, K. & Coghill, R. Evidence against pain specificity in the dorsal posterior insula. F1000Res. 4, 362 (2015).
Palermo, S., Benedetti, F., Costa, T. & Amanzio, M. Pain anticipation: an activation likelihood estimation meta-analysis of brain imaging studies. Hum. Brain Mapp. 36, 1648–1661 (2015).
Brown, C.A. & Jones, A.K.P. A role for midcingulate cortex in the interruptive effects of pain anticipation on attention. Clin. Neurophysiol. 119, 2370–2379 (2008).
Wiech, K. et al. Anterior insula integrates information about salience into perceptual decisions about pain. J. Neurosci. 30, 16324–16331 (2010).
Misra, G. & Coombes, S.A. Neuroimaging evidence of motor control and pain processing in the human midcingulate cortex. Cereb. Cortex 25, 1906–1919 (2015).
Baliki, M.N., Geha, P.Y. & Apkarian, A.V. Parsing pain perception between nociceptive representation and magnitude estimation. J. Neurophysiol. 101, 875–887 (2009).
Cai, Y.Q., Wang, W., Hou, Y.Y. & Pan, Z.Z. Optogenetic activation of brainstem serotonergic neurons induces persistent pain sensitization. Mol. Pain 10, 70 (2014).
Ringkamp, M. & Raja, S.N. A sore spot: central or peripheral generation of chronic neuropathic spontaneous pain? Pain 155, 1189–1191 (2014).
Dittgen, T. et al. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc. Natl. Acad. Sci. USA 101, 18206–18211 (2004).
Tang, W. et al. Faithful expression of multiple proteins via 2A-peptide self-processing: a versatile and reliable method for manipulating brain circuits. J. Neurosci. 29, 8621–8629 (2009).
Smith, R.H., Levy, J.R. & Kotin, R.M. A simplified baculovirus-AAV expression vector system coupled with one-step affinity purification yields high-titer rAAV stocks from insect cells. Mol. Ther. 17, 1888–1896 (2009).
During, M.J., Young, D., Baer, K., Lawlor, P. & Klugmann, M. Development and optimization of adeno-associated virus vector transfer into the central nervous system. Methods Mol. Med. 76, 221–236 (2003).
Paxinos, G. & Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates (Academic, 2001).
Fillinger, C., Yalcin, I., Barrot, M. & Veinante, P. Afferents to anterior cingulate areas 24a and 24b and midcingulate areas 24a′ and 24b′ in the mouse. Brain Struct. Funct. 222, 1509–1532 (2017).
Njoo, C., Heinl, C. & Kuner, R. In vivo siRNA transfection and gene knockdown in spinal cord via rapid noninvasive lumbar intrathecal injections in mice. J. Vis. Exp. 85, 51229 (2014).
Barthas, F. et al. The anterior cingulate cortex is a critical hub for pain-induced depression. Biol. Psychiatry 77, 236–245 (2015).
Tadel, F., Baillet, S., Mosher, J.C., Pantazis, D. & Leahy, R.M. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 879716 (2011).
Acknowledgements
We thank R. LeFaucheur for secretarial help, as well as N. Gehrig, V. Buchert, L. Brenner, H.-J. Wrede, D. Baumgartl-Ahlert and K. Meyer for technical assistance. We are grateful to the Interdisciplinary Neurobehavioral Core Facility in Heidelberg for support with behavioral experiments. We gratefully acknowledge funding in form of SFB1158 grants from the Deutsche Forschungsgemeinschaft (DFG) to R.K. (project B01), T.K. (project B08), R.S. (project A05) and H.F. (project B07), European Research Council (ERC) Advanced Investigator grants to R.K. (Pain Plasticity 294293) and H.F. (Phantommind 230249) and DFG funding via the Excellence Cluster CellNetworks (Ectop funding to R.K. and H.F.). We acknowledge support from the European Molecular Biology Organization (EMBO) to L.L.T. in the form of an EMBO long-term postdoctoral fellowship.
Author information
Authors and Affiliations
Contributions
L.L.T., R.S., H.F., T.K. and R.K. were involved in manuscript preparation. L.L.T. conducted the experiments and analyzed data. R.K. designed the study and wrote the manuscript. W.T. generated the viruses; V.G. helped with behavioral experiments; C.H. and P.P. performed electrophysiology experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Atlas representations of the midcingulate (MCC) region targeted in this study compared against the anterior cingulate (ACC) region commonly reported.
Coronal sections are shown on the upper panels. High magnification images of the outlined areas depicting the cingulate areas Cg1 and Cg2 of the MCC versus the ACC are shown on the right. The sagittal representation of the MCC (blue) versus the ACC (gray) is shown in the lower panel. Dotted areas represent the MCC region targeted in this study and the ACC regions commonly reported in previous studies16, 27, 30. The segregation between the MCC and ACC are based upon recent anatomical reports7, 51. Atlas images modified from Paxinos and Franklin, 2001.
Supplementary Figure 2 Cellular properties of opsin- and control GFP-expressing neurons obtained from patch clamp whole-cell recordings performed in slices from the MCC.
Individual data points are plotted for (a) membrane resting potential, (b) membrane resistance, (c) action potential threshold, (d) action potential amplitude, (e) 50 % spike width and (f) after-hyperpolarization obtained from ChR2- (blue, n = 9), ArchT- (red, n = 20) and GFP-expressing (green, n = 22) cells from a total of 6 mice. Median values are indicated with black lines. None of the recorded parameters differed between the groups. p > 0.05 as indicated in panels, Kruskal-Wallis test.
Supplementary Figure 3 Lack of phototoxicity in morphological analyses on opsin-expressing cortical sections.
(a) Representative images for TUNEL staining (red) in ArchT-expressing (green) sections after in vivo photo-stimulation (top panel). The area (300 x 300 μm2) surrounding the fiber tract site (dotted line) was quantified for positive TUNEL staining. In the positive control, TUNELpositive staining (red) was observed after pre-treatment with DNase (middle panel). No TUNEL positive staining was observed in the negative control (lower panel). Nuclei are stained with DAPI (blue). Scalebar, 100 μm. (b) Quantification of the TUNEL-positive area in a 300 x 300 μm2 cortical region below the fiber tract. ArchT-expressing animals that received photosilencing in vivo showed a significantly lower area of TUNEL expression compared to the positive control and was comparable with the negative control (n = 2 mice/group; positive vs negative: t = 15.737, p < 0.001; positive vs ArchT: t = 14.847, p < 0.001; negative vs ArchT: t = 0.891, p = 1; between groups: F(2,6) = 156.292, p < 0.001). (c) Representative images of stained Nissl bodies from cortical tissue of ArchT-negative and ArchTexpressing animals after in vivo photo-illumination (n = 4 mice). The fiber tract is denoted by dashed lines in the examples shown. Scalebar, 100 μm. (d) Quantification of the average Nissl body counts within a 300 x 300 μm2 area surrounding the fiber tract in ArchT-negative and ArchT-positive tissue after in vivo photo-illumination were compared (n = 8 samples for ArchT-negative, n = 13 samples for ArchT-positive from a total of 6 mice). No differences were detected between the two groups (t = -1.853, p = 0.08). Error bars in boxplots (b, d) represent 10th and 90th percentile, cross lines indicate median values, *p < 0.05, one-way ANOVA in b, t-test in d
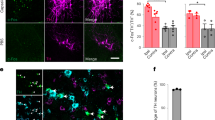
Supplementary Figure 4 Fos expression in the MCC after capsaicin injection in the hindpaw.
(a) Immunofluorescence images from the black dotted boxed region (in atlas representation on the left) are shown. Examples depict Fos expression (green) in capsaicin-injected (upper panel) and non-injected (middle panel) mice as well as a negative control (lower panel). Nuclei are stained with DAPI (blue). Scale bar, 200 μm. (b) Example of suppression of unilateral intraplantar capsaicin-evoked Fos expression (red) by yellow light (YL) photo-illumination in ArchT-expressing cells (green) in the MCC (upper panels) and in the absence of YL (lower panels). ArchT-expressing cells positive for Fos expression are indicated with unfilled arrows, ArchT-expressing cells negative for Fos are indicated with filled arrows. Scale bar, 50 μm.
Supplementary Figure 5 Optogenetic modulation of activity in the MCC and/or the hind limb region of the primary somatosensory cortex (S1HL) on capsaicin-evoked nocifensive behaviors and basal mechanical sensitivities of the hindpaws.
(a) Acute nocifensive behaviors evoked by intraplantar capsaicin injection into the paw contralateral to the ArchT-expressing hemisphere upon yellow light (YL) exposure in the MCC (n = 5 for control-implanted, n = 6 for ArchT; df = 9, t = 0.103, p = 0.92), S1HL (n = 5 for control-implanted, n = 6 for ArchT; df = 9, t = 0.529, p = 0.61) or both the contralateral MCC and S1HL simultaneously (n = 5 per group; df = 8, t = -0.71, p = 0.433). (b) Effects of cortical photo-silencing on basal mechanical sensitivity (in the absence of capsaicin treatment) of the hindpaws of animals expressing ArchT either in the left MCC (n = 5; contralateral groups x force F(10,40) = 1.961, p = 0.07; ipsilateral groups x force F(10,40) = 0.818, p = 0.613), left S1HL (n = 8; contralateral groups x force F(10,70) = 1.315, p = 0.24; ipsilateral groups x force F(10,70) = 1.594, p = 0.127), or both the left MCC and S1HL (n = 6; contralateral groups x force F(10,50) = 2.159, p = 0.036; ipsilateral groups x force F(10,50) = 1.188, p = 0.322). (c) The 40% mechanical threshold values are shown (contralateral groups x treatment F(4,32) = 3.115, p = 0.028; ipsilateral groups x treatment F(4,32) = 0.769, p = 0.553). Error bars in boxplots (panel a) represent 10th and 90th percentile, cross lines indicate median values. t-test in panel a or two-way ANOVA with repeated measures with Bonferroni multiple comparison in panels b and c. *p < 0.05 compared to baseline; p values in each panel represents significance between entire stimulus-response curves; n.s., not significant.
Supplementary Figure 6 Effects of cortical illumination on paw mechanical sensitivity of control animals.
(a) The experimental scheme is shown. Control mice were implanted as indicated, but neither received viral injections nor were photo-illuminated (S1HL: blue circles and bars, n = 7; MCC: black triangles and bars, n = 9). Another MCC control implanted group (gray triangles and bars, n = 9) and GFP control group (injected with rAAV-CaMKII-GFP and implanted, green triangles and bars, n = 5) received identical photo-illumination after capsaicin was injected in the flank (pre-cap groups F(4,20) = 0.657, p = 0.623; 15 mins groups F(4,20) = 0.477, p = 0.753; 45 mins groups F(4,20) = 0.196, p = 0.94). (b) The 40% mechanical threshold values are shown (groups x treatment F(8,75) = 1.251, p = 0.282). *p < 0.05 compared to baseline, two-way ANOVA with Bonferroni multiple comparison (upper) or one-way ANOVA (lower); n.s., not significant.
Supplementary Figure 7 Effects of silencing the MCC or hind limb area of the primary somatosensory (S1HL) activity on capsaicin-evoked secondary mechanical hypersensitivity in the paw.
(a) Effects of contralateral yellow light (yellow bars) exposure in the MCC or S1HL on capsaicin-evoked secondary mechanical hyperalgesia to von Frey filament application on the injected paw, according to the schematic outline in the upper panel (n = 9 for MCC control, n = 10 for MCC ArchT, n = 7 for S1HL control, n = 9 for S1HL ArchT; MCC: pre-cap groups F(1,5) = 3.152, p = 0.08; 15 mins groups F(1,5) = 47.772, p < 0.001; 45 mins groups F(1,5) = 33.634, p < 0.001; S1HL: pre-cap groups F(1,5) = 0.00714, p = 0.933; 15 mins groups F(1,5) = 9.864, p = 0.002; 45 mins groups F(1,5) = 0.0805, p = 0.777). The 40% mechanical threshold values are shown in panel b (groups F(3,6) = 9.608, p < 0.001, groups x treatment F(6,93) = 3.063, p = 0.009). (c) Firing rate of a representative cell responding to capsaicin with a significant increase in spike frequency. Spikes per second are shown for 15 minutes recording during baseline, 15 min after injection of capsaicin and with capsaicin coupled with yellow light (YL; df = 2, H = 1235.956, p < 0.001). *p < 0.05 compared to baseline, †p < 0.05 compared between groups in panel a, †p < 0.05 compared with MCC ArchT group (red) in panel b, two-way ANOVA with Bonferroni multiple comparison; p values in each panel represents significance between entire stimulus-response curves; n.s., not significant. Error bars in boxplots (c) represent 10th and 90th percentile, cross lines indicate median values, one-way ANOVA on Ranks with Tukey post-hoc test.
Supplementary Figure 8 Effects of optogenetic inhibition and activation in the MCC on paw mechanical withdrawal responses.
(a) A schematic timeline of experimental manipulations and behavioral von Frey response measurements is shown. (b) The 40% mechanical thresholds are plotted to compare the impact of silencing MCC activity over the induction phase (blue), during ongoing measurements (red) or just prior to the measurement of the late phase (orange) of capsaicin-evoked mechanical hypersensitivity (n = 9 for black, n = 9 in blue, n = 10 in red, n = 6 in orange; groups F(3,6) = 10.204, p < 0.001; groups x treatment F(6,90) = 3.604, p = 0.003). (c) Typical example of a neuron in a whole-cell slice recording showing action potentials evoked by ChR2 stimulation via blue light (blue bars above trace) and their silencing by simultaneous ArchT stimulation via yellow light (yellow bar) in MCC pyramidal neurons co-expressing ArchT and ChR2. (d) Frequency response curves showing ArchT-mediated suppression of capsaicin-evoked mechanical hypersensitivity can be partially reversed by direct ChR2-mediated excitation of MCC neurons over the delayed phase (n = 10 for red, n = 9 for black, n = 9 for gray, n = 11 for blue; pre-cap groups F(3,15) = 1.457, p = 0.227; 15 mins groups F(3,15) = 30.424, p < 0.001; 45 mins groups F(3,15) = 16.214, p < 0.001). In panel b, *p < 0.05 compared to respective baseline, †p < 0.05 compared with control group (black), two-way ANOVA with Bonferroni multiple comparison. In panel d, *p < 0.05 compared to respective baseline, †p < 0.05 as compared with ArchT group (red triangles), two-way ANOVA with Bonferroni multiple comparison. Color-coded p values in panel d represent significance between entire stimulus-response curves; n.s., not significant.
Supplementary Figure 9 Functional characterization of rAVV-CaMKII-ChR2 expression in the cortex.
(a) Exclusion of ChR2 (red) expression from GABAergic (green) inhibitory neurons in the MCC. Nuclei is shown with DAPI staining (blue), scale bar 100 μm. (b) Quantification of action potentials evoked by direct blue light stimulation (at different intensities) of MCC neurons expressing ChR2 in a graded manner proportional to stimulus intensity and frequency (n = 4 cells per group from 4 mice; frequency F(3, 9) = 7.815, p = 0.007, intensity F(3, 9) = 54.318, p < 0.001). (c) High magnification example of co-localization of ChR2 and Fos expression post-stimulation of MCC in vivo; scale bar, 50 μm. In panel b, *p < 0.05 compared to group receiving lowest intensity (black), †p < 0.05 compared to respective 10 Hz stimulation, two-way ANOVA with Bonferroni multiple comparison.
Supplementary Figure 10 Effects of MCC stimulation on mechanical withdrawal responses in hindpaws.
Mechanical hypersensitivity to von Frey filament application of the (a) contralateral and (b) ipsilateral paws during direct stimulation of the MCC at 10 - 30 Hz frequencies evident in stimulus-response curves (n = 6 for control, n = 7 for ChR2 group; contralateral groups: baseline F(1,5) = 3.196, p = 0.80, 10 Hz F(1,5) = 7.322, p = 0.009, 20 Hz F(1,5) = 8.833, p = 0.004, 30 Hz F(1,5) = 35.198, p < 0.001; ipsilateral groups: baseline F(1,5) = 0.0597, p = 0.808, 10 Hz F(1,5) = 12.31, p < 0.001, 20 Hz F(1,5) = 19.117, p < 0.001, 30 Hz F(1,5) = 49.25, p < 0.001). (c) Long-lasting mechanical hypersensitivity upon unilateral MCC stimulation (n = 13 for baseline, n = 13 for BL, n = 7 for 4 hr, n = 10 for 24 hr; contralateral treatment F(3,15) = 17.404, p < 0.001; ipsilateral treatment F(3,15) = 7.68, p < 0.001). *p < 0.05 compared to respective baseline, †p < 0.05 compared between groups, two-way ANOVA with Bonferroni multiple comparison. Color-coded p values in each panel represent significance between entire stimulus-response curves; n.s., not significant.
Supplementary Figure 11 Quantification of Fos-positive cells in various cortical regions.
In the left column, a group of ChR2-expressing animals (gray) received no photo-illumination while another group of ChR2-expressing animals (red) received 15 min blue light (BL) photo-stimulation in vivo. In the right column, a group of ArchT-expressing animals (gray) received only a capsaicin injection while another group of ArchT-expressing animals (red) animals received 15 min yellow light (YL) photo-illumination in vivo after capsaicin was injected. Fos-positive counts from both left and right hemispheres were compared in the PrL (n = 19 samples from 3 mice; BL: Uleft = 40, p = 0.293, Uright = 39, p = 0.531; YL: Uleft = 0, p = 0.003, Uright = 2, p = 0.009), IL (n = 9 samples from 2 mice; BL: Uleft = 18.5, p = 0.64, Uright = 16, p = 1; YL: Uleft = 0, p = 0.003, Uright = 0, p = 0.036), claustrum (n = 27 samples from 4 mice; BL: Uleft = 43.5, p = 0.038, Uright = 27, p = 0.013; YL: Uleft = 140.5, p = 0.032, Uright = 103, p = 0.281), S1HL (n = 13 samples from 3 mice; BL: Uleft = 8, p = 0.003, Uright = 0, p = 0.036; YL: Uleft = 7, p < 0.001, Uright = 0, p < 0.001) and CeA (n = 9 samples from 3 mice; BL: Uleft = 11, p = 0.313, Uright = 9, p = 0.202; YL: Uleft = 11, p = 0.006, Uright = 8.5, p = 0.497). Error bars in all boxplots represent 10th and 90th percentile, cross lines indicate median values. Prelimbic, PrL; infralimbic, IL; primary somatosensory hind limb region, S1HL; central amygdala, CeA. *p < 0.05 compared to control group, Mann-Whitney test.
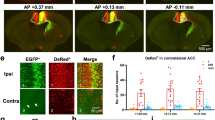
Supplementary Figure 12 Example of Fos downregulation in the PI upon inhibition of the MCC in the capsaicin model.
Fos expression (red) in the PI and claustrum in capsaicin-injected ArchT-expressing mice in the absence (upper panels) or presence of yellow light (YL) photo-inhibition in the MCC (lower panels). Nuclei are stained with DAPI (blue). Scale bar, 100 μm.
Supplementary Figure 13 Quantification and examples of Fos expression in the thalamus upon manipulation of activity in the MCC.
Quantification of Fos expression in the medial thalamus (MD) in the absence and presence of blue light (BL) in the MCC of ChR2-expressing mice or yellow light (YL) in ArchT-expressing mice (post-capsaicin injection) are shown in boxplots in the upper right panels (n = 5 samples per BL group, n = 11 samples per YL group from a total of 3 mice; UBL = 11, p = 0.31; UYL = 33, p = 0.39). Positive Fos expression was observed in the paraventricular thalamus (PV) in all groups and shown here as positive controls (n = 4 samples per BL group, n = 7 samples per YL group; UBL = 3, p = 0.2; UYL = 20, p = 0.95). Example of a negative control (only secondary antibody) is shown in the lower panel. Fos expression is shown in red, nuclei are stained with DAPI (blue). Scale bars, 100 μm. Error bars in all boxplots represent 10th and 90th percentile, cross lines indicate median values. p > 0.05, Mann-Whitney test.
Supplementary Figure 14 Mechanical behavioral responses of hindpaws upon silencing activity of the MCC–PI pathway or MCC–NAc pathway, or in the PI directly.
(a) Basal mechanical sensitivities (in the absence of capsaicin treatment) of both paws of mice injected with rAAV-CaMKII-eArchT or rAAV-CaMKII-GFP in the MCC and photo-silenced with yellow light (YL) in the ipsilateral PI (n = 8 for MCC-PI eArchT, contralateral treatment F(2,14) = 0.949, p = 0.411; ipsilateral treatment F(2,14) = 1.191, p = 0.333; n = 5 for MCC-PI GFP, contralateral treatment F(2,8) = 0.234, p = 0.796; ipsilateral treatment F(2,14) = 0.444, p = 0.656) or silenced in the ipsilateral N.Ac. (n = 6 for MCC-N.Ac. eArchT, contralateral treatment F(2,10) = 0.363, p = 0.704; ipsilateral treatment F(2,10) = 0.274, p = 0.766; n = 6 for MCC-N.Ac. GFP, contralateral treatment F(2,10) = 1.522, p = 0.265; ipsilateral treatment F(2,10) = 0.0323, p = 0.968). (b) Basal mechanical sensitivities of both paws of mice (in the absence of capsaicin treatment) injected with rAAV-CaMKII-eArchT in the left PI and in the absence and presence of photo-silencing with YL (n = 10; contralateral treatment F(2,18) = 1.111, p = 0.351; ipsilateral treatment F(2,18) = 0.0751, p = 0.928). Throughout the figure, *p < 0.05 compared to baseline, †p < 0.05 compared between groups, two-way ANOVA repeated measures with Bonferroni multiple comparison; p values in each panel represents significance between each stimulus curve as indicated in panels; n.s., not significant.
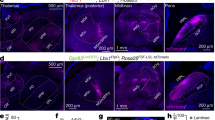
Supplementary Figure 15 Viral tracing examples showing projections of excitatory neurons from the PI within the raphe nucleus (RMg) regions.
(a) The PI region is outlined in green in the atlas schematic on the left. Corresponding confocal images (boxed region number 1) showing the site of injection and expression of GFP confined within the PI are shown with and without nuclei staining (DAPI in blue); scale bar, 500 μm. Higher magnification images of GFP-expressing cells within the insert box (number 2) is depicted in the lower panels, scale bar 250 μm. (b) Virally-traced fiber tracts (green) traced from excitatory neurons in the PI (shown in panel a above) are observed in the RMg, scale bar, 250 μm. High magnification images of the RMg region (indicated with asterisk) are shown in the lower panels, 100 μm. Nuclei staining are shown with DAPI (blue). py, pyramidal tract; Tz, nucleus of the trapezoid body; ml, medial lemniscus; Ppy, parapyramidal nucleus; RMg, raphe magnus nucleus; Rpa, raphe pallidus nucleus; Gi, gigantocellular reticular nucleus; GiA, gigantocellular reticular nucleus, alpha part.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 (PDF 3213 kb)
Rights and permissions
About this article
Cite this article
Tan, L., Pelzer, P., Heinl, C. et al. A pathway from midcingulate cortex to posterior insula gates nociceptive hypersensitivity. Nat Neurosci 20, 1591–1601 (2017). https://doi.org/10.1038/nn.4645
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4645
This article is cited by
-
Anterior cingulate cross-hemispheric inhibition via the claustrum resolves painful sensory conflict
Communications Biology (2024)
-
Inhibitory insula-ACC projections modulate affective but not sensory aspects of neuropathic pain
Molecular Brain (2023)
-
First-in-human prediction of chronic pain state using intracranial neural biomarkers
Nature Neuroscience (2023)
-
Social circuits and their dysfunction in autism spectrum disorder
Molecular Psychiatry (2023)
-
Prefrontal engrams of long-term fear memory perpetuate pain perception
Nature Neuroscience (2023)