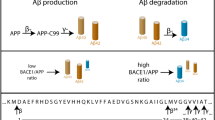
Abstract
Cleavage of amyloid precursor protein (APP) by BACE-1 (β-site APP cleaving enzyme-1) is the rate-limiting step in amyloid-β (Aβ) production and a neuropathologic hallmark of Alzheimer's disease; thus, physical approximation of this substrate-enzyme pair is a crucial event with broad biological and therapeutic implications. Despite much research, neuronal locales of APP and BACE-1 convergence and APP cleavage remain unclear. Here we report an optical assay, based on fluorescence complementation, for visualizing in cellulo APP–BACE-1 interactions as a simple on/off signal. Combining this with other assays tracking the fate of internalized APP in hippocampal neurons, we found that APP and BACE-1 interacted in both biosynthetic and endocytic compartments, particularly along recycling microdomains such as dendritic spines and presynaptic boutons. In axons, APP and BACE-1 were cotransported, and they also interacted during transit. Finally, our assay revealed that the Alzheimer's disease–protective 'Icelandic' mutation greatly attenuates APP–BACE-1 interactions, suggesting a mechanistic basis for protection. Collectively, the data challenge canonical models and provide concrete insights into long-standing controversies in the field.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
O'Brien, R.J. & Wong, P.C. Amyloid precursor protein processing and Alzheimer's disease. Annu. Rev. Neurosci. 34, 185–204 (2011).
Rajendran, L. & Annaert, W. Membrane trafficking pathways in Alzheimer's disease. Traffic 13, 759–770 (2012).
Kinoshita, A. et al. Demonstration by FRET of BACE interaction with the amyloid precursor protein at the cell surface and in early endosomes. J. Cell Sci. 116, 3339–3346 (2003).
Sannerud, R. et al. ADP ribosylation factor 6 (ARF6) controls amyloid precursor protein (APP) processing by mediating the endosomal sorting of BACE1. Proc. Natl. Acad. Sci. USA 108, E559–E568 (2011).
Greenfield, J.P. et al. Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer β-amyloid peptides. Proc. Natl. Acad. Sci. USA 96, 742–747 (1999).
Prasad, H. & Rao, R. The Na+/H+ exchanger NHE6 modulates endosomal pH to control processing of amyloid precursor protein in a cell culture model of Alzheimer disease. J. Biol. Chem. 290, 5311–5327 (2015).
Das, U. et al. Activity-induced convergence of APP and BACE-1 in acidic microdomains via an endocytosis-dependent pathway. Neuron 79, 447–460 (2013).
Kerppola, T.K. Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells. Nat. Protoc. 1, 1278–1286 (2006).
de Virgilio, M., Kiosses, W.B. & Shattil, S.J. Proximal, selective, and dynamic interactions between integrin αIIbβ3 and protein tyrosine kinases in living cells. J. Cell Biol. 165, 305–311 (2004).
Remy, I., Montmarquette, A. & Michnick, S.W. PKB/Akt modulates TGF-β signalling through a direct interaction with Smad3. Nat. Cell Biol. 6, 358–365 (2004).
Citron, M., Teplow, D.B. & Selkoe, D.J. Generation of amyloid β protein from its precursor is sequence specific. Neuron 14, 661–670 (1995).
Sahlin, C. et al. The Arctic Alzheimer mutation favors intracellular amyloid-beta production by making amyloid precursor protein less available to alpha-secretase. J. Neurochem. 101, 854–862 (2007).
Muratore, C.R. et al. The familial Alzheimer's disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Hum. Mol. Genet. 23, 3523–3536 (2014).
Small, S.A. & Gandy, S. Sorting through the cell biology of Alzheimer's disease: intracellular pathways to pathogenesis. Neuron 52, 15–31 (2006).
Kaech, S., Huang, C.F. & Banker, G. Short-term high-resolution imaging of developing hippocampal neurons in culture. Cold Spring Harb. Protoc. 2012, 340–343 (2012).
El Meskini, R., Cline, L.B., Eipper, B.A. & Ronnett, G.V. The developmentally regulated expression of Menkes protein ATP7A suggests a role in axon extension and synaptogenesis. Dev. Neurosci. 27, 333–348 (2005).
Buggia-Prévot, V. et al. A function for EHD family proteins in unidirectional retrograde dendritic transport of BACE1 and Alzheimer's disease Aβ production. Cell Reports 5, 1552–1563 (2013).
Koo, E.H. & Squazzo, S.L. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J. Biol. Chem. 269, 17386–17389 (1994).
Perez, R.G. et al. Mutagenesis identifies new signals for β-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Aβ42. J. Biol. Chem. 274, 18851–18856 (1999).
Knowles, M.K. et al. Single secretory granules of live cells recruit syntaxin-1 and synaptosomal associated protein 25 (SNAP-25) in large copy numbers. Proc. Natl. Acad. Sci. USA 107, 20810–20815 (2010).
Wang, L. et al. α-Synuclein multimers cluster synaptic vesicles and attenuate recycling. Curr. Biol. 24, 2319–2326 (2014).
Gitler, D. et al. Molecular determinants of synapsin targeting to presynaptic terminals. J. Neurosci. 24, 3711–3720 (2004).
Washbourne, P., Bennett, J.E. & McAllister, A.K. Rapid recruitment of NMDA receptor transport packets to nascent synapses. Nat. Neurosci. 5, 751–759 (2002).
Sekine-Aizawa, Y. & Huganir, R.L. Imaging of receptor trafficking by using α-bungarotoxin-binding-site-tagged receptors. Proc. Natl. Acad. Sci. USA 101, 17114–17119 (2004).
Jonsson, T. et al. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature 488, 96–99 (2012).
Benilova, I. et al. The Alzheimer disease protective mutation A2T modulates kinetic and thermodynamic properties of amyloid-β (Aβ) aggregation. J. Biol. Chem. 289, 30977–30989 (2014).
Maloney, J.A. et al. Molecular mechanisms of Alzheimer disease protection by the A673T allele of amyloid precursor protein. J. Biol. Chem. 289, 30990–31000 (2014).
Rajendran, L. et al. Alzheimer's disease beta-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. USA 103, 11172–11177 (2006).
Choy, R.W., Cheng, Z. & Schekman, R. Amyloid precursor protein (APP) traffics from the cell surface via endosomes for amyloid β (Aβ) production in the trans-Golgi network. Proc. Natl. Acad. Sci. USA 109, E2077–E2082 (2012).
Park, M. et al. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron 52, 817–830 (2006).
Wang, Z. et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell 135, 535–548 (2008).
Yap, C.C. & Winckler, B. Harnessing the power of the endosome to regulate neural development. Neuron 74, 440–451 (2012).
Vassar, R., Kovacs, D.M., Yan, R. & Wong, P.C. The beta-secretase enzyme BACE in health and Alzheimer's disease: regulation, cell biology, function, and therapeutic potential. J. Neurosci. 29, 12787–12794 (2009).
Buxbaum, J.D. et al. Alzheimer amyloid protein precursor in the rat hippocampus: transport and processing through the perforant path. J. Neurosci. 18, 9629–9637 (1998).
Lazarov, O., Lee, M., Peterson, D.A. & Sisodia, S.S. Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J. Neurosci. 22, 9785–9793 (2002).
Cirrito, J.R. et al. Endocytosis is required for synaptic activity-dependent release of amyloid-β in vivo. Neuron 58, 42–51 (2008).
DeBoer, S.R., Dolios, G., Wang, R. & Sisodia, S.S. Differential release of beta-amyloid from dendrite- versus axon-targeted APP. J. Neurosci. 34, 12313–12327 (2014).
Kandalepas, P.C. et al. The Alzheimer's beta-secretase BACE1 localizes to normal presynaptic terminals and to dystrophic presynaptic terminals surrounding amyloid plaques. Acta Neuropathol. 126, 329–352 (2013).
Kamal, A., Almenar-Queralt, A., LeBlanc, J.F., Roberts, E.A. & Goldstein, L.S. Kinesin-mediated axonal transport of a membrane compartment containing beta-secretase and presenilin-1 requires APP. Nature 414, 643–648 (2001).
Lazarov, O. et al. Axonal transport, amyloid precursor protein, kinesin-1, and the processing apparatus: revisited. J. Neurosci. 25, 2386–2395 (2005).
Szodorai, A. et al. APP anterograde transport requires Rab3A GTPase activity for assembly of the transport vesicle. J. Neurosci. 29, 14534–14544 (2009).
Sisodia, S.S. A cargo receptor mystery APParently solved? Science 295, 805–807 (2002).
Goldsbury, C. et al. Inhibition of APP trafficking by tau protein does not increase the generation of amyloid-beta peptides. Traffic 7, 873–888 (2006).
Ring, S. et al. The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J. Neurosci. 27, 7817–7826 (2007).
Tang, Y. et al. Early and selective impairments in axonal transport kinetics of synaptic cargoes induced by soluble amyloid beta-protein oligomers. Traffic 13, 681–693 (2012).
Ganguly, A. & Roy, S. Using photoactivatable GFP to track axonal transport kinetics. Methods Mol. Biol. 1148, 203–215 (2014).
Acknowledgements
We thank G. Banker (Oregon Health and Science University) for the NPYss:mCherry and TfR:mCherry/GFP constructs, G. Patterson and J. Lipincott-Schwartz (NIH) for the GalT:mCherry/GFP constructs, Z.-H. Sheng (NIH) for the synaptophysin-mRFP construct, W. Almers (Oregon Health and Science University) for the broken CMV promoter and D. Boehning (The University of Texas Health Science Center) for the APP(Lon):GFP construct. Constructs from Addgene and investigators are acknowledged in the Online Methods. U.D. was partly supported by a pilot award from the UCSD Alzheimer's Center (P50 AG005131). This work was supported by grants from the NIH/NIA (R01AG048218 and NIH/NINDS (R01NS075233) to S.R.
Author information
Authors and Affiliations
Contributions
U.D. and S.R. designed the assays and wrote the paper. U.D. designed and performed most of the experiments and data analysis. L.W. designed and performed the synaptic targeting and some of the axonal transport experiments. A.G. and L.W. helped with neuronal cultures and J.M.S. performed and analyzed some of the axonal transport experiments. S.L.W. and E.H.K. provided key reagents and technical advice.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Expression levels of various constructs used in this study.
(a) Neurons were transfected with the indicated plasmids and soluble mCherry (volume filler), fixed ~ 5-6 h posttransfection, and immunostained with a polyclonal anti-GFP antibody (that detects all VN/VC fragments, visualized using an anti-Rabbit-647 antibody). Note comparable expression with all expressed proteins, including the various APP mutants.
(b) HEK293T cells were co-transfected with APP:VN and BACE-1:VC (incubated with or without the γ-secretase inhibitor BMS-299897) and analyzed by Western blotting with two anti-APP antibodies. Note that omission of BMS-299897 led to a shift in the ~ 30kDa band (β-cleavage product of APP:VN, red arrowheads) to ~ 26kDa (blue arrowheads). This 4kDa shift is likely due to further cleavage of the APP:VN-β-CTF into a ~ 26kDa AICD-VN fragment and a ~ 4kDa Aβ fragment (which will be likely secreted). Also note that in these gels, a ~ 11kDa band is greatly attenuated upon omission of BMS- 299897. This fragment probably represents endogenous β-CTF’s – generated from the endogenous APP present in these cells – and upon BMS-299897 removal, these are further cleaved into Aβ that is secreted (the ~ 6kDa endogenous AICD probably turns over too rapidly in the absence of BMS-299897). These results further confirm that APP:VN is cleaved by BACE-1:VC in the OptiCAB assay. Also note holo-APP is better recognized by the CT15 antibody (brackets).
Supplementary Figure 2 Complementation of familial AD mutants and experiments with ‘crippled CMV promoter’.
(a) BACE-1 null fibroblasts were co-transfected with BACE-1:VC and WT-APP:VN (or VN-tagged APP Arctic and London mutations) Note that with both mutants there is an increase in levels of the ~ 30kDa fragment likely corresponding to bCTF:VN (as noted by other studies 12, 13); though there was no change in the levels of mutant-APP/BACE-1 complementation in neurons after ~ 5-6h (b).
(c) Left: APP:VN and BACE-1:VC were sub-cloned into “crippled” CMV promoters (ΔAPP:VN and ΔBACE:VC), known to express lower protein-levels (see “methods”; note that ~ 48h of expression was necessary to visualize fluorescence by light microscopy). Right: Untransfected or transfected neurons (constructs indicates on X-axis) were fixed and immunostained with an anti-APP antibody (A4) to detect total APP fluorescence in soma (Y-axis). Note that fluorescence-levels in ΔAPP:VN/ΔBACE:VC are only slightly higher than untransfected neurons.
(d) ΔAPP:VN/ΔBACE-1:VC transfected neurons were either incubated with Alexa-transferrin (or co-transfected with the stated organelle markers), and imaged as described in “methods”. Note colocalization of ΔAPP/ΔBACE-1 BifC with
endosomes.
Supplementary Figure 3 Identity of axonal APP and BACE-1 carriers.
(a,b) Neurons were co-transfected with APP:GFP (or BACE-1:GFP) and RFP-tagged endocytic markers (Rab5 and Rab11) as indicated; and imaged live by simultaneous dual-cam imaging. Note little co-transport of APP or BACE-1 with endocytic markers.
Supplementary Figure 4 Internalization of APP in neuronal soma.
(a, b) Neurons were co-transfected with BBS-APP and various organelle markers, incubated with BTX-594, and internalized APP was evaluated in cell bodies by colocalization analyses (see ‘methods’). Colocalization of internalized BTX-594 with organelle markers was as follows: TfR (41.6 %), Rab5 (49.9), GalT (44.1), NPYss (31.2 %), and LAMP-1 (79.1 %). 10-18 neurons from two separate cultures were analyzed; * p < 0.05.
Supplementary Figure 5 Trafficking parameters of protective APP(Ice) mutant.
(a) Construct design and experimental strategy to evaluate internalization of APP.
(b) Similar internalization of WT and APP(Ice) mutants in neurons, quantified on right (26-28 neurons from three independent experiments were analyzed).
(c) Comparing vesicle transport kinetics of APP WT and APP(Ice) in dendrites. Neurons were transfected with APP:GFP or APP(Ice):GFP, and were imaged live with fast image acquisition (1 frame/sec for 200 secs), 5-6 h post-transfection. Note that various transport parameters – frequency, velocity and run length – are largely similar between the two groups, though a slight decrease in anterograde particle frequency was seen with APP(Ice) mutant. ~ 450 particles from 28-36 neurons – from three separate experiments – were analyzed; * p < 0.05.
Supplementary Figure 6 OptiCAB assay in HEK293T cell lines.
(a) HEK293T cells were transfected with indicated constructs and visualized 5-6 h post-transfection. Note in the APP:VN/BACE-1:VC group, complemented APP/BACE-1 fluorescence was widespread and punctate (zoomed inset). However, diminished fluorescence was seen in in APP:VN/BACE-1:VC transfected cells treated with an inhibitor of clathrin-dependent endocytosis (Dynasore), or in cells transfected with APPYENPTY:VN/BACE-1:VC. Fluorescence complementation was also markedly attenuated when cells were transfected with APP(Ice):VN/BACE-1:VC.
(b)Western blot of HEK293T cells transfected with APP:VN/BACE-1:VC (in presence of a γ-secretase inhibitor) shows the expected ~30 kDa β-cleavage product of APP:VN (data from 2-3 independent cultures).
Supplementary Figure 7 Neuronal recycling endosomes are acidic.
Neurons were co-transfected with TfR:pHluorin and soluble mCherry for ~5-6 h and imaged live. TfR:pHluorin is the recycling endosome marker (TfR) tagged to a pHsensitive probe (pHluorin). Note that the TfR:pHluorin fluorescence increases upon incubation in an alkaline media – indicating that these vesicles are acidic (middle panel) – and quenches in an acidic media (bottom panel), with little change in the soluble mCherry fluorescence (right panels).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Table 1 (PDF 1537 kb)
Rights and permissions
About this article
Cite this article
Das, U., Wang, L., Ganguly, A. et al. Visualizing APP and BACE-1 approximation in neurons yields insight into the amyloidogenic pathway. Nat Neurosci 19, 55–64 (2016). https://doi.org/10.1038/nn.4188
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4188
This article is cited by
-
Physiological Roles of β-amyloid in Regulating Synaptic Function: Implications for AD Pathophysiology
Neuroscience Bulletin (2023)
-
APP-BACE1 Interaction and Intracellular Localization Regulate Aβ Production in iPSC-Derived Cortical Neurons
Cellular and Molecular Neurobiology (2023)
-
Spatial snapshots of amyloid precursor protein intramembrane processing via early endosome proteomics
Nature Communications (2022)
-
Enhanced cleavage of APP by co-expressed Bace1 alters the distribution of APP and its fragments in neuronal and non-neuronal cells
Molecular Neurobiology (2022)
-
The Alzheimer’s gene SORL1 is a regulator of endosomal traffic and recycling in human neurons
Cellular and Molecular Life Sciences (2022)