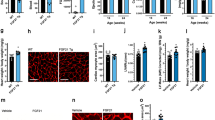
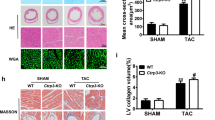
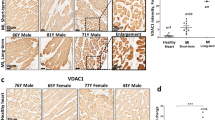
Abstract
Patients with diabetes and other obesity-linked conditions have increased susceptibility to cardiovascular disorders1. The adipocytokine adiponectin is decreased in patients with obesity-linked diseases2. Here, we found that pressure overload in adiponectin-deficient mice resulted in enhanced concentric cardiac hypertrophy and increased mortality that was associated with increased extracellular signal-regulated kinase (ERK) and diminished AMP-activated protein kinase (AMPK) signaling in the myocardium. Adenovirus-mediated supplemention of adiponectin attenuated cardiac hypertrophy in response to pressure overload in adiponectin-deficient, wild-type and diabetic db/db mice. In cultures of cardiac myocytes, adiponectin activated AMPK and inhibited agonist-stimulated hypertrophy and ERK activation. Transduction with a dominant-negative form of AMPK reversed these effects, suggesting that adiponectin inhibits hypertrophic signaling in the myocardium through activation of AMPK signaling. Adiponectin may have utility for the treatment of hypertrophic cardiomyopathy associated with diabetes and other obesity-related diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Reilly, M.P. & Rader, D.J. The metabolic syndrome: more than the sum of its parts? Circulation 108, 1546–1551 (2003).
Ouchi, N., Kihara, S., Funahashi, T., Matsuzawa, Y. & Walsh, K. Obesity, adiponectin and vascular inflammatory disease. Curr. Opin. Lipidol. 14, 561–566 (2003).
Ilercil, A. et al. Relationship of impaired glucose tolerance to left ventricular structure and function: The Strong Heart Study. Am. Heart J. 141, 992–998 (2001).
Rutter, M.K. et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation 107, 448–454 (2003).
Schannwell, C.M., Schneppenheim, M., Perings, S., Plehn, G. & Strauer, B.E. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology 98, 33–39 (2002).
Kahn, B.B. & Flier, J.S. Obesity and insulin resistance. J. Clin. Invest. 106, 473–481 (2000).
Scherer, P.E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 (1995).
Maeda, N. et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 8, 731–737 (2002).
Kubota, N. et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J. Biol. Chem. 277, 25863–25866 (2002).
Shibata, R. et al. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J. Biol. Chem. 279, 28670–28674 (2004).
Xiao, L. et al. MEK1/2–ERK1/2 mediates alpha1-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J. Mol. Cell. Cardiol. 33, 779–787 (2001).
Esposito, G. et al. Cardiac overexpression of a G(q) inhibitor blocks induction of extracellular signal-regulated kinase and c-Jun NH(2)-terminal kinase activity in in vivo pressure overload. Circulation 103, 1453–1458 (2001).
Kobayashi, H. et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ. Res. 94, e27–e31 (2004).
Tomas, E. et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc. Natl. Acad. Sci. USA 99, 16309–16313 (2002).
Yamauchi, T. et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 8, 1288–1295 (2002).
Wu, X. et al. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes 52, 1355–1363 (2003).
Ouchi, N. et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem. 279, 1304–1309 (2004).
Chan, A.Y., Soltys, C.L., Young, M.E., Proud, C.G. & Dyck, J.R. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J. Biol. Chem. 279, 32771–32179 (2004).
Nagata, D., Mogi, M. & Walsh, K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J. Biol. Chem. 278, 31000–31006 (2003).
Mu, J., Brozinick, J.T., Jr, Valladares, O., Bucan, M. & Birnbaum, M.J. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell 7, 1085–1094 (2001).
Kudo, N., Barr, A.J., Barr, R.L., Desai, S. & Lopaschuk, G.D. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J. Biol. Chem. 270, 17513–17520 (1995).
Tian, R., Musi, N., D'Agostino, J., Hirshman, M.F. & Goodyear, L.J. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation 104, 1664–1669 (2001).
Pimentel, D.R. et al. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ. Res. 89, 453–460 (2001).
Rogers, J.H. et al. RGS4 causes increased mortality and reduced cardiac hypertrophy in response to pressure overload. J. Clin. Invest. 104, 567–576 (1999).
Acknowledgements
We acknowledge the technical assistance of S. Tanaka and A. Bialik. This work was supported by US National Institutes of Health (NIH) grants HL66957, AR40197, AG15052 and AG17241 to K. Walsh, HL61639 and HL20612 to W.S. Colucci, NIH Cardiovascular Scientist Training Grant HL07224 to D.R. Pimentel; and grants from the Japanese Ministry of Education and the Japan Society for Promotion of Science-Research for the Future Program. R. Shibata, N. Ouchi and M. Ito were supported by grants from the Uehara Memorial Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Body weight, hemodynamic and echocardiographic measurements in wild-type and APN-KO mice at 7 days post-surgery. (PDF 289 kb)
Rights and permissions
About this article
Cite this article
Shibata, R., Ouchi, N., Ito, M. et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med 10, 1384–1389 (2004). https://doi.org/10.1038/nm1137
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1137
This article is cited by
-
Caloric restriction reduces sympathetic activity similar to beta-blockers but conveys additional mitochondrio-protective effects in aged myocardium
Scientific Reports (2021)
-
Heal the heart through gut (hormone) ghrelin: a potential player to combat heart failure
Heart Failure Reviews (2021)
-
Effects of Adiponectin on Diastolic Function in Mice Underwent Transverse Aorta Constriction
Journal of Cardiovascular Translational Research (2020)
-
Cardiac hypertrophy with obesity is augmented after pregnancy in C57BL/6 mice
Biology of Sex Differences (2019)
-
Pioglitazone strengthen therapeutic effect of adipose-derived regenerative cells against ischemic cardiomyopathy through enhanced expression of adiponectin and modulation of macrophage phenotype
Cardiovascular Diabetology (2019)