Abstract
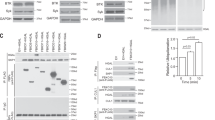
Binding of antigen to the B cell receptor induces a calcium response, which is required for proliferation and antibody production. CD22, a B cell surface protein, inhibits this signal through mechanisms that have been obscure. We report here that CD22 augments calcium efflux after B cell receptor crosslinking. Inhibition of plasma membrane calcium-ATPase (PMCA) attenuated these effects, as did disruption by homologous recombination of the gene encoding PMCA4a and PMCA4b. PMCA coimmunoprecipitated with CD22 in an activation-dependent way. CD22 cytoplasmic tyrosine residues were required for association with PMCA and enhancement of calcium efflux. Moreover, CD22 regulation of efflux and the calcium response required the tyrosine phosphatase SHP-1. Thus, SHP-1 and PMCA provide a mechanism by which CD22, a tissue-specific negative regulator, can affect calcium responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
Change history
16 May 2004
appended aop PDF with erratum PDF (will be corrected for print issue), and placed footnote in XML at all occurrences of Figure 5
Notes
*Note: In the version of this article originally published online, the key for Figure 5c and d was labeled incorrectly. The correct labels are "SHIPKO empty" and "SHIPKO CD22+." This error has been corrected for the HTML and print versions of this article.
References
Winslow, M.M., Neilson, J.R. & Crabtree, G.R. Calcium signalling in lymphocytes. Curr. Opin. Immunol. 15, 299–307 (2003).
Taylor, C.W. Controlling calcium entry. Cell 111, 767–769 (2002).
Lewis, R.S. Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol. 19, 497–521 (2001).
Snitsarev, V.A. & Taylor, C.W. Overshooting cytosolic Ca2+ signals evoked by capacitative Ca2+ entry result from delayed stimulation of a plasma membrane Ca2+ pump. Cell Calcium 25, 409–417 (1999).
Klishin, A., Sedova, M. & Blatter, L.A. Time-dependent modulation of capacitative Ca2+ entry signals by plasma membrane Ca2+ pump in endothelium. Am. J. Physiol. 274, C1117–1128 (1998).
Scharff, O., Foder, B. & Skibsted, U. Hysteretic activation of the Ca2+ pump revealed by calcium transients in human red cells. Biochim. Biophys. Acta 730, 295–305 (1983).
Sato, S. et al. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity 5, 551–562 (1996).
Otipoby, K.L. et al. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature 384, 634–637 (1996).
O'Keefe, T.L., Williams, G.T., Batista, F.D. & Neuberger, M.S. Deficiency in CD22, a B cell-specific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. J. Exp. Med. 189, 1307–1313 (1999).
O'Keefe, T.L., Williams, G.T., Davies, S.L. & Neuberger, M.S. Hyperresponsive B cells in CD22-deficient mice. Science 274, 798–801 (1996).
Nitschke, L., Carsetti, R., Ocker, B., Kohler, G. & Lamers, M.C. CD22 is a negative regulator of B-cell receptor signalling. Curr. Biol. 7, 133–143 (1997).
Nadler, M.J., McLean, P.A., Neel, B.G. & Wortis, H.H. B cell antigen receptor-evoked calcium influx is enhanced in CD22-deficient B cell lines. J. Immunol. 159, 4233–4243 (1997).
Schulte, R.J., Campbell, M.A., Fischer, W.H. & Sefton, B.M. Tyrosine phosphorylation of CD22 during B cell activation. Science 258, 1001–1004 (1992).
Wilson, G.L., Fox, C.H., Fauci, A.S. & Kehrl, J.H. cDNA cloning of the B cell membrane protein CD22: a mediator of B-B cell interactions. J. Exp. Med. 173, 137–146 (1991).
Doody, G.M. et al. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science 269, 242–244 (1995).
Campbell, M.A. & Klinman, N.R. Phosphotyrosine-dependent association between CD22 and protein tyrosine phosphatase 1C. Eur. J. Immunol. 25, 1573–1579 (1995).
Ono, M. et al. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell 90, 293–301 (1997).
Blasioli, J., Paust, S. & Thomas, M.L. Definition of the sites of interaction between the protein tyrosine phosphatase SHP-1 and CD22. J. Biol. Chem. 274, 2303–2307 (1999).
Poe, J.C., Fujimoto, M., Jansen, P.J., Miller, A.S. & Tedder, T.F. CD22 forms a quaternary complex with SHIP, Grb2, and Shc. A pathway for regulation of B lymphocyte antigen receptor-induced calcium flux. J. Biol. Chem. 275, 17420–17427 (2000).
Hashimoto, A., Hirose, K., Okada, H., Kurosaki, T. & Iino, M. Inhibitory modulation of B cell receptor-mediated Ca2+ mobilization by Src homology 2 domain-containing inositol 5′-phosphatase (SHIP). J. Biol. Chem. 274, 11203–11208 (1999).
Jin, L., McLean, P.A., Neel, B.G. & Wortis, H.H. Sialic acid binding domains of CD22 are required for negative regulation of B cell receptor signaling. J. Exp. Med. 195, 1199–1205 (2002).
Strehler, E.E. & Zacharias, D.A. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol. Rev. 81, 21–50 (2001).
Philipson, K.D. & Nicoll, D.A. Sodium-calcium exchange: a molecular perspective. Annu. Rev. Physiol. 62, 111–133 (2000).
Herscher, C.J. & Rega, A.F. On the mechanism of inhibition of the PMCa2+-ATPase by lanthanum. Ann. NY Acad. Sci. 834, 407–409 (1997).
Chen, J. et al. Autocrine action and its underlying mechanism of nitric oxide on intracellular Ca2+ homeostasis in vascular endothelial cells. J. Biol. Chem. 275, 28739–28749 (2000).
DiPolo, R. Ca pump driven by ATP in squid axons. Nature 274, 390–392 (1978).
Otipoby, K.L., Draves, K.E. & Clark, E.A. CD22 regulates B cell receptor-mediated signals via two domains that independently recruit Grb2 and SHP-1. J. Biol. Chem. 276, 44315–44322 (2001).
Lajas, A.I., Sierra, V., Camello, P.J., Salido, G.M. & Pariente, J.A. Vanadate inhibits the calcium extrusion in rat pancreatic acinar cells. Cell Signal. 13, 451–456 (2001).
Zhang, B.X., Zhao, H., Loessberg, P. & Muallem, S. Activation of the plasma membrane Ca2+ pump during agonist stimulation of pancreatic acini. J. Biol. Chem. 267, 15419–15425 (1992).
Thastrup, O., Cullen, P.J., Drobak, B.K., Hanley, M.R. & Dawson, A.P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl. Acad. Sci. USA 87, 2466–2470 (1990).
Balasubramanyam, M. & Gardner, J.P. Protein kinase C modulates cytosolic free calcium by stimulating calcium pump activity in Jurkat T cells. Cell Calcium 18, 526–541 (1995).
Brandt, P., Neve, R.L., Kammesheidt, A., Rhoads, R.E. & Vanaman, T.C. Analysis of the tissue-specific distribution of mRNAs encoding the plasma membrane calcium-pumping ATPases and characterization of an alternately spliced form of PMCA4 at the cDNA and genomic levels. J. Biol. Chem. 267, 4376–4385 (1992).
Liu, B.F., Xu, X., Fridman, R., Muallem, S. & Kuo, T.H. Consequences of functional expression of the plasma membrane Ca2+ pump isoform 1a. J. Biol. Chem. 271, 5536–5544 (1996).
Filoteo, A.G. et al. Plasma membrane Ca2+ pump in rat brain. Patterns of alternative splices seen by isoform-specific antibodies. J. Biol. Chem. 272, 23741–23747 (1997).
Caride, A.J., Filoteo, A.G., Enyedi, A., Verma, A.K. & Penniston, J.T. Detection of isoform 4 of the plasma membrane calcium pump in human tissues by using isoform-specific monoclonal antibodies. Biochem. J. 316, 353–359 (1996).
Magyar, C.E., White, K.E., Rojas, R., Apodaca, G. & Friedman, P.A. Plasma membrane Ca2+-ATPase and NCX1 Na+/Ca2+ exchanger expression in distal convoluted tubule cells. Am. J. Physiol. Renal Physiol. 283, F29–40 (2002).
Schatzmann, H.J. ATP-dependent Ca++-extrusion from human red cells. Experientia 22, 364–365 (1966).
Dean, W.L., Chen, D., Brandt, P.C. & Vanaman, T.C. Regulation of platelet plasma membrane Ca2+-ATPase by cAMP-dependent and tyrosine phosphorylation. J. Biol. Chem. 272, 15113–15119 (1997).
Sanders, D., Brownlee, C. & Harper, J.F. Communicating with calcium. Plant Cell 11, 691–706 (1999).
Bautista, D.M., Hoth, M. & Lewis, R.S. Enhancement of calcium signalling dynamics and stability by delayed modulation of the plasma-membrane calcium-ATPase in human T cells. J. Physiol. 541, 877–894 (2002).
Monteith, G.R., Wanigasekara, Y. & Roufogalis, B.D. The plasma membrane calcium pump, its role and regulation: new complexities and possibilities. J. Pharmacol. Toxicol. Meth. 40, 183–190 (1998).
Moller, J.V., Juul, B. & le Maire, M. Structural organization, ion transport, and energy transduction of P-type ATPases. Biochim. Biophys. Acta 1286, 1–51 (1996).
Resink, T.J. et al. Platelet calcium-linked abnormalities in essential hypertension. Ann. NY Acad. Sci. 488, 252–265 (1986).
Donnadieu, E., Bismuth, G. & Trautmann, A. Calcium fluxes in T lymphocytes. J. Biol. Chem. 267, 25864–25872 (1992).
Balasubramanyam, M., Kimura, M., Aviv, A. & Gardner, J.P. Kinetics of calcium transport across the lymphocyte plasma membrane. Am. J. Physiol. 265, C321–327 (1993).
Carafoli, E. Biogenesis: plasma membrane calcium ATPase: 15 years of work on the purified enzyme. FASEB J. 8, 993–1002 (1994).
Rapp, U.R. et al. Rapid induction of hemopoietic neoplasms in newborn mice by a rafmil/myc recombinant murine retrovirus. J. Virol. 55, 23–33 (1985).
Winding, P. & Berchtold, M.W. The chicken B cell line DT40: a novel tool for gene disruption experiments. J. Immunol. Meth. 249, 1–16 (2001).
Maeda, A., Kurosaki, M., Ono, M., Takai, T. & Kurosaki, T. Requirement of SH2-containing protein tyrosine phosphatases SHP-1 and SHP-2 for paired immunoglobulin-like receptor B (PIR-B)-mediated inhibitory signal. J. Exp. Med. 187, 1355–1360 (1998).
Takata, M. et al. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 13, 1341–1349 (1994).
Acknowledgements
We thank R. Berland, K. Dunlap, E. Herrera, L. Jin, T. Imanishi-Kari and N. Rosenberg for discussions; E.A. Clark, D.L. Gill, M. Kurosaki and T. Kurosaki for reagents; and T. Trombley for help with some of the experiments. Supported by the National Institutes of Health (H.H.W., G.E.S. and B.G.N.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chen, J., McLean, P., Neel, B. et al. CD22 attenuates calcium signaling by potentiating plasma membrane calcium-ATPase activity. Nat Immunol 5, 651–657 (2004). https://doi.org/10.1038/ni1072
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1072
This article is cited by
-
Ginsenoside Rg-1 prevents elevated cytosolic Ca2+ via store-operated Ca2+ entry in high-glucose–stimulated vascular endothelial and smooth muscle cells
BMC Complementary Medicine and Therapies (2022)
-
Calcium-dependent signalling in B-cell lymphomas
Oncogene (2021)
-
Calcium signalling in T cells
Nature Reviews Immunology (2019)
-
Galectin-9 suppresses B cell receptor signaling and is regulated by I-branching of N-glycans
Nature Communications (2018)
-
The role of CD22 and Siglec-G in B-cell tolerance and autoimmune disease
Nature Reviews Rheumatology (2014)