Abstract
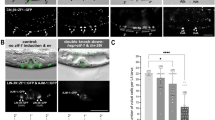
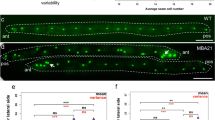
Cell-fate specification and cell-cell signaling have been well studied during vulva development in Caenorhabditis elegans and provide a paradigm in evolutionary developmental biology1,2. Pristionchus pacificus has been developed as a 'satellite' organism with an integrated physical and genetic map that allows detailed comparisons to C. elegans3,4,5. A common aspect of vulva formation in both species is the polarization of the P7.p lineage, which is responsible for vulval symmetry. In C. elegans, Wnt signaling is crucial for P7.p cell-fate patterning6; nothing is known about vulval symmetry in P. pacificus. We isolated mutations that disrupt polarization of the P7.p lineage in P. pacificus and found that the corresponding gene encodes a Frizzled-like molecule. In addition, mutations in Ppa-lin-17 (encoding Frizzled) and morpholino knock-down of Ppa-lin-44 (encoding Wnt), Ppa-egl-20 (encoding Wnt), Ppa-mig-5 (encoding Dsh), Ppa-apr-1 (encoding APC) and Ppa-bar-1 (encoding β-catenin) results in gonad-independent vulva differentiation, indicating that these genes have a role in a negative signaling process. In contrast, in C. elegans, Wnt signaling has a positive role in vulva induction, and mutations in bar-1 result in a hypoinduced phenotype7. Therefore, whereas the molecular mechanisms that generate vulval symmetry are conserved, the genetic control of vulva induction diversified during evolution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
References
Wang, M. & Sternberg, P.W. Pattern formation during C. elegans vulval induction. Curr. Top. Dev. Biol. 51, 189–220 (2001).
Sommer, R.J. As good as they get: cells in nematode vulva development and evolution. Curr. Opin. Cell. Biol. 13, 715–720 (2001).
Eizinger, A. & Sommer, R.J. The homeotic gene lin-39 and the evolution of nematode epidermal cell fates. Science 278, 452–455 (1997).
Simpson, P. Evolution of development in closely related species of flies and worms. Nat. Rev. Genet. 3, 907–917 (2002).
Srinivasan, J. et al. An integrated physical and genetic map of the nematode Pristionchus pacificus. Mol. Genet. Genomics 269, 715–722 (2003).
Inoue, T. et al. C. elegans LIN-18 is a Ryk ortholog and functions in parallel to LIN-17/Frizzled in Wnt signaling. Cell 118, 795–806 (2004).
Eisenmann, D.M., Maloof, J.N., Simske, J.S., Kenyon, C. & Kim, S.K. The beta-catenin homolog BAR-1 and LET-60 Ras coordinately regulate the Hox gene lin-39 during Caenorhabditis elegans vulval development. Development 125, 3667–3680 (1998).
Lambshead, P.J.D. Recent developments in marine benthic biodiversity research. Oceanis 19, 5–24 (1993).
Pires-DaSilva, A. & Sommer, R J. Conservation of the global sex determination gene tra-1 in distantly related nematodes. Genes Dev. 18, 1198–1208 (2004).
Hill, R.J. & Sternberg, P.W. The gene lin-3 encodes an inductive signal for vulval development in C. elegans. Nature 358, 470–476 (1992).
Chen, N. & Greenwald, I. The lateral signal for LIN-12/Notch in C. elegans vulval development comprises redundant secreted and transmembrane DSL proteins. Dev. Cell 6, 183–192 (2004).
Ceol, C.J. & Horvitz, H.R. dpl-1 DP and efl-1 E2F act with lin-35 Rb to antagonize Ras signaling in C. elegans vulval development. Mol. Cell 7, 461–473 (2001).
Sommer, R.J. & Sternberg, P.W. Apoptosis and change of competence limit the size of the vulva equivalence group in Pristionchus pacificus: a genetic analysis. Curr. Biol. 6, 52–59 (1996).
Sigrist, C.B. & Sommer, R.J. Vulva formation in Pristionchus pacificus relies on continuous gonadal induction. Dev. Genes Evol. 209, 451–459 (1999).
Kimble, J. Alterations in cell lineage following laser ablation of cells in the somatic gonad of Caenorhabditis elegans. Dev. Biol. 87, 286–300 (1981).
Jungblut, B. & Sommer, R.J. Novel cell-cell interactions during vulva development in Pristionchus pacificus. Development 127, 3295–3303 (2000).
Sawa, H., Lobel, L. & Horvitz, H.R. The Caenorhabditis elegans gene lin-17, which is required for certain asymmetric cell divisions, encodes a putative seven-transmembrane protein similar to the Drosophila frizzled protein. Genes Dev. 10, 2189–2197 (1996).
Ceol, C.J. & Horvitz, H.R. A new class of C. elegans synMuv genes implicates a Tip60/NuA4-like HAT complex as a negative regulator of Ras signaling. Dev. Cell 6, 563–576 (2004).
Maloof, J.N., Whangbo, J., Harris, J.M., Jongeward, G.D. & Kenyon, C. A Wnt signaling pathway controls hox gene expression and neuroblast migration in C. elegans. Development 126, 37–49 (1999).
Sommer, R.J. et al. The Pristionchus HOX gene Ppa-lin-39 inhibits programmed cell death to specify the vulva equivalence group and is not required during vulval induction. Development 125, 3865–3873 (1998).
Acknowledgements
We thank A. Gutierrez and J. Srinivasan for help with the original cloning of Ppa-lin-44, Ppa-egl-20, Ppa-mig-5 and Ppa-apr-1; A. Rojas-Munoz for the zebrafish morpholino; R. Hong, A. Pires-daSilva, D. Rudel and A. Streit for critically reading the manuscript; and members of the laboratory of R.J.S. for discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Amino acid sequence comparison of egl-20, lin-44, mig-5, apr-1, bar-1 and lin-18 between P. pacificus and C. elegans. (PDF 5328 kb)
Rights and permissions
About this article
Cite this article
Zheng, M., Messerschmidt, D., Jungblut, B. et al. Conservation and diversification of Wnt signaling function during the evolution of nematode vulva development. Nat Genet 37, 300–304 (2005). https://doi.org/10.1038/ng1512
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1512
This article is cited by
-
Opinion: What do rescue experiments with heterologous proteins tell us and what not?
Parasitology Research (2022)
-
The Same or Not the Same: Lineage-Specific Gene Expansions and Homology Relationships in Multigene Families in Nematodes
Journal of Molecular Evolution (2015)
-
Divergent gene expression in the conserved dauer stage of the nematodes Pristionchus pacificus and Caenorhabditis elegans
BMC Genomics (2012)
-
The Pristionchus pacificus genome provides a unique perspective on nematode lifestyle and parasitism
Nature Genetics (2008)
-
Phylogeny of the nematode genus Pristionchus and implications for biodiversity, biogeography and the evolution of hermaphroditism
BMC Evolutionary Biology (2007)