Abstract
Spermatogenesis is a complex process that involves cooperation of germ cells and testicular somatic cells. Various genetic disorders lead to impaired spermatogenesis, defective sperm function and male infertility1. Here we show that Cnot7−/− males are sterile owing to oligo-astheno-teratozoospermia, suggesting that Cnot7, a CCR4-associated transcriptional cofactor2, is essential for spermatogenesis. Maturation of spermatids is unsynchronized and impaired in seminiferous tubules of Cnot7−/− mice. Transplantation of spermatogonial stem cells from male Cnot7−/− mice to seminiferous tubules of Kit mutant mice (KitW/W-v) restores spermatogenesis, suggesting that the function of testicular somatic cells is damaged in the Cnot7−/− condition. The testicular phenotypes of Cnot7−/− mice are similar to those of mice deficient in retinoid X receptor beta (Rxrb)3. We further show that Cnot7 binds the AF-1 domain of Rxrb and that Rxrb malfunctions in the absence of Cnot7. Therefore, Cnot7 seems to function as a coregulator of Rxrb in testicular somatic cells and is thus involved in spermatogenesis.
Similar content being viewed by others
Main
Genetic analyses in yeast suggest that CAF1, a component of the CCR4-NOT complex, has multiple roles in regulating transcription4. In addition, proteins in the CCR4-NOT complex are involved in mRNA metabolism in yeast5,6. Two mammalian homologs of yeast CAF1 are known: Cnot7 (also called CAF1) and Cnot8 (also called CALIF)7,8. Both are expressed in a variety of tissues, with relatively high expression of Cnot7 in lung, liver and thyroid gland7,8. Cnot7 interacts with members of the TOB-BTG antiproliferative family, which comprises Tob1, Tob2, Btg1, Btg2 (also called PC3 and TIS21), Btg3 (also called ANA) and Btg4 (also called PC3B; refs. 7,9,10). The proteins of this family are implicated in regulation of transcription11,12,13,14.
To elucidate the physiological role of mammalian Cnot7, we produced mutant mice lacking the gene Cnot7 by means of homologous recombination (Fig. 1a–d). Homozygous Cnot7 knockout (Cnot7−/−) mice were alive at birth and born at the predicted mendelian frequency. Adult Cnot7−/− mice had normal health, size and behavior, except that male Cnot7−/− mice were sterile. Cnot7+/− males had normal fertility and Cnot7−/− females produced average-size litters (6.3 ± 2.1 offspring per litter; n = 14 litters).
(a) Schematic diagram of the targeting vector and the wild-type and targeted alleles. Black boxes show exons 2, 3 and 4 of Cnot7. nLacZ, lacZ coding sequence with nuclear localization signal; neo, neomycin resistance gene driven by the thymidine kinase promoter; DT-A, diphtheria toxin A-chain gene; RV, EcoRV; Nc, NcoI; K, KpnI; Xh, XhoI. (b) The 5′ external probe was used for Southern-blot analysis to detect homologous recombination. Tail DNAs from F1 progeny of heterozygote intercrosses were digested with NcoI and subjected to Southern-blot hybridization. (c) Northern-blot analysis of total RNAs (10 μg) from testes of Cnot7+/+, Cnot7+/− and Cnot7−/− mice with the 701-bp fragment (nucleotides 158–858) of the human CNOT7 cDNA. (d) Western-blot analysis of protein extracts from Cnot7+/+, Cnot7+/− and Cnot7−/− MEFs with antibodies to Cnot7. (e) Comparison of testis weight in three 5-month-old Cnot7+/− and Cnot7−/− mice. The organs were trimmed free of fat and weighed. (f,g) Number and motility of sperm prepared from three 5-month-old Cnot7+/− and Cnot7−/− mice. Sperm counts (f) and percentages of motile sperm (g) were determined visually by microscopy. Error bars represent s.d. Statistical significance (*P < 0.01) in each assay was assessed by Student's t-test. (h) Morphology of spermatozoa. Spermatozoa were collected from the cauda epididymides of Cnot7−/− mice and photographed without fixation under Nomarski optics (magnification, ×600). (i) Transmission electron micrographs of spermatozoa from the cauda epididymides in male Cnot7+/+ (top) and Cnot7−/− (middle and bottom) mice. In Cnot7+/+ mice, head was normally hooked in shape and nucleus (Nu) was condensed. Acrosome (Ac) and mitochondria (Mt) were also normally formed. In Cnot7−/− mice, nuclei of spermatozoa were condensed but did not form the characteristic hooked shape. Acrosomes were expanded and attached to nucleus. Abnormal arrangement and localization of mitochondria were apparent and flagella (F) were incorrectly attached. The middle panel shows localization of condensed nucleus in the cytoplasm compartments. The differences in body weight, sperm count, motility and morphology between Cnot7+/+ and Cnot7+/− mice were not significant. Scale bars, 1 μm.
There were no gross anatomical differences in the seminal vesicles and prostates among Cnot7+/+, Cnot7+/− and Cnot7−/− males, but the testes of Cnot7−/− mice were smaller than those of Cnot7+/+ or Cnot7+/− mice (Fig. 1e). Cnot7−/− mice produced only 7% as much sperm as Cnot7+/+ or Cnot7+/− mice (Fig. 1f), and their spermatozoa beat less vigorously and generated less forward momentum (Fig. 1g). Almost all spermatozoa from Cnot7−/− mice had irregularly shaped heads, abnormally arranged mitochondria and erroneously attached flagella (Fig. 1h,i). Electron microscopic analysis detected ultrastructural components, such as condensed chromatin, acrosomes and flagella, including axoneme, mitochondrial sheath, outer dense fibers and fibrous sheath, in spermatozoa from Cnot7−/− mice. But their arrangements were irregular and maturation of sperm was abnormal (Fig. 1i). The degree of morphological irregularity varied: spermatozoa of Cnot7−/− mice were round-headed (73%), tapered-headed (20%), symplast (5%) or nearly normal (2%). We also observed malformation of spermatids in the seminiferous tubules (see Fig. 3d). Taken together, these data indicate that oligo-astheno-teratozoospermia (low sperm number and motility and abnormal sperm morphology) underlies the sterility of Cnot7−/− males.
(a) Expression of Cnot7 in testicular cells. β-galactosidase staining of testes from Cnot7+/− mice indicates strong Cnot7 expression in somatic cells. Arrows and arrowheads indicate Leydig and Sertoli cells, respectively. Scale bar, 50 μm. (b) A testis from a transplanted recipient mouse showing β-galactosidase expression. β-galactosidase-positive (blue) stretches of seminiferous tubules are colonies from donor spermatogonial stem cells. (c) Histological section of KitW/W-v recipient seminiferous tubules stained with periodic acid–Schiff and hematoxylin. Arrow and arrowhead indicate colonized and noncolonized seminiferous tubules, respectively, after transplantation. Scale bar, 50 μm. (d) Transmission electron micrographs showing a malformed head of elongating spermatid in testis of a Cnot7−/− mouse (upper panel) and a normally formed head of elongating spermatid obtained from the transplantation experiment (lower panel). Nu, condensed nuclei; Ac, acrosome. Scale bars, 1 μm. (e) Histological section of KitW/W-v recipient seminiferous tubules stained with toluidine blue. Scale bar, 10 μm.
To further analyze sperm competence for fertilization, we carried out in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) experiments. For the IVF experiment, we freed oocytes from cumulus cells and the zona pellucida, because epididymal spermatozoa of Cnot7−/− mice had poor motility and were unable to penetrate these egg layers. Even under these experimental conditions, spermatozoa of Cnot7−/− mice hardly fertilized oocytes, whereas Cnot7+/− spermatozoa had normal fertilizing ability (Table 1). Direct injection of spermatids from Cnot7−/− mice into oocytes by conventional ICSI resulted in normal fertilization and production of pups after embryo transfer. The efficiencies were comparable to those after ICSI using control spermatids (Table 1), indicating that spermatids from Cnot7−/− mice had an adequate set of haploid genome and full oocyte-activating capacity. We conclude from these data that Cnot7−/− male germ cells have defects in postmeiotic modifications that are essential for enabling natural delivery of the paternal genome into the oocytes.
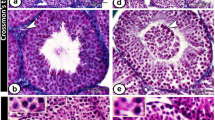
Detailed histological analysis of seminiferous tubules from Cnot7−/− mice showed a reduction in the number of late spermatids and the presence of large, round, clear vacuoles (Fig. 2a–g). In severe cases, most germ cells were lost in seminiferous tubules of Cnot7−/− mice (Fig. 2f). We often observed multiple generations of elongated spermatids in the same section of seminiferous tubules of Cnot7−/− mice (Fig. 2h). The data indicate that maturation of spermatids in Cnot7−/− mice is unsynchronized. Spermatogenesis in Cnot7−/− mice was also distinguished from that in control mice by the presence of syncytial (multinucleated) germ cells (Fig. 2h) and a greater number of apoptotic cells (Fig. 2i,j). Lipids were unusually accumulated in the cytoplasm of Sertoli cells of adult Cnot7−/− mice as compared with control mice (Fig. 2c,g,k,l), suggesting that deficiency of Cnot7 induces a metabolic defect in Sertoli cells.
(a,b,e,f) Histological sections of seminiferous tubules prepared from 5-month-old Cnot7+/− (a,b) and Cnot7−/− (e,f) males were stained with hematoxylin and eosin. Asterisks indicate typical vacuoles. Scale bars: a,e, 100 μm; b,f, 50 μm. (c,d,g,h) Impairment of sperm maturation in seminiferous tubules of Cnot7−/− mice. Histological sections of seminiferous tubules prepared from 2-month-old Cnot7+/− (c,d) and Cnot7−/− (g,h) males were stained with toluidine blue. Asterisks, arrow and filled arrowheads indicate vacuoles, a syncytial cell and unusual accumulations of lipids in the cytoplasm of Sertoli cells, respectively. Spermatogenesis was well-synchronized in Cnot7+/− males at stage VIII (c) and at stage XII (d) but was not well-synchronized in Cnot7−/− males at stage VIII (g) and at stage XII (h), which featured multiple generations of spermatogenic cells (open arrowheads). Double arrowheads in d and h indicate secondary spermatocytes. Scale bars, 25 μm. (i,j) More germ cell apoptosis in testes of Cnot7−/− mice. Apoptotic cells in seminiferous tubules from 5-month-old Cnot7+/− (i) and Cnot7−/− (j) males were stained (brown) intensively by TUNEL. Arrows indicate apoptotic cells. Scale bars, 50 μm. (k,l) Unusual accumulation of lipids (arrowheads) in the cytoplasm of Sertoli cells. Light micrograph showing spermatogenesis in testis from a Cnot7−/− mouse was stained with toluidine blue (k). Transmission electron micrograph also shows a lipid droplet in Sertoli cells of testis from a Cnot7−/− mouse (l). Scale bars: k, 10 μm; l, 2 μm.
β-galactosidase staining of seminiferous tubules from Cnot7+/− mice showed that Cnot7 was strongly expressed in Sertoli and Leydig cells (Fig. 3a) but only weakly expressed in early round spermatids, spermatocytes and spermatogonia. Because intimate interactions between germ cells and somatic support cells are essential for spermatogenesis, the impaired spermatogenesis in Cnot7−/− mice may be caused by defects intrinsic to the support cells. To examine this possibility, we carried out spermatogonial stem cell transplantation15,16. We transplanted germ cells from 8- to 10-week-old Cnot7−/− males into the seminiferous tubules of WBB6F1 W/Wv (KitW/W-v) mice, busulfan-treated nude (BALB/c) mice or irradiated C57BL/6 mice. The testes of these mice are hospitable for donor cell colonization because they lack endogenous germ cells and have functionally normal Sertoli cells15. By tracing expression of the lacZ transgene, we found that the transplanted Cnot7−/− spermatogonial stem cells differentiated into spermatids in the seminiferous tubules of recipient mice after transplantation (Fig. 3b,c). Morphologically normal spermatids (Fig. 3d) were aligned at the luminal side of seminiferous tubules in the KitW/W-v recipient testes by 3 months after transplantation (Fig. 3c,e). These results suggest that Cnot7 functions in testicular somatic cells to establish the proper testicular microenvironment for spermatogenesis. Results of reciprocal transplantation experiments, in which germ cells from transgenic mice carrying the pCXN-eGFP transgene17 were transmitted into the seminiferous tubules of busulfan-treated Cnot7−/− mice, were consistent with that conclusion (data not shown).
Several nuclear receptors including estrogen receptor α, androgen receptor, retinoic acid receptor α (Rara) and Rxrb are essential for spermatogenesis3,18,19,20. Of these, Rxrb is expressed in Sertoli and Leydig cells3,21. Rxrb−/− male mice are sterile, owing to abnormal germ cell maturation that leads to oligo-astheno-teratozoospermia. In addition, Sertoli cells of Rxrb−/− mice undergo a progressive accumulation of lipids3.
We expressed FLAG-tagged human CNOT7 in COS7 cells together with Rxrb or other nuclear receptors (Rxra, Rxrg, Rara and vitamin D receptor) fused to glutathione S-transferase (GST). GST pull-down assays with the cell lysates showed that CNOT7 interacted only with GST-Rxrb (Fig. 4a). This interaction was confirmed by immunoprecipitation experiments with lysates of TTE3 Sertoli cells22 (Fig. 4b). Rxra, Rxrb and Rxrg share a conserved DNA-binding domain (∼95% homology) and C-terminal ligand-binding domain (AF-2, ∼87% homology). In contrast, the sequence of the N-terminal domain (AF-1), which is involved in autonomous ligand-independent transcriptional activation, is divergent across family members23. GST pull-down assays showed that the AF-1 domain of Rxrb interacted with CNOT7 whereas the DNA-binding and AF-2 domains did not (Fig. 4c).
(a,c) FLAG-tagged CNOT7 was coexpressed in COS7 cells with GST-fused nuclear receptors (NRs; mouse Rxra, mouse Rxrb, mouse Rxrg, mouse Rara and rat vitamin D receptor (Vdr); a) or GST-fused distinct portions of Rxrb (c). Total protein extracts were subjected to GST pull-down assay followed by immunoblotting (IB) with monoclonal antibody against FLAG (top panel). The lower two panels show expression of each indicated protein. (b) Proteins in the lysates of TTE3 Sertoli cells were immunoprecipitated with antibodies to Cnot7 (αCnot7) or nonspecific IgG. The immunoprecipitates were subjected to immunoblotting (IB) with antibodies to Rxrb (upper panel) or to Cnot7 (lower panel). Filled arrowheads to the left indicate bands that interacted specifically with antibodies; open arrowheads to the right indicate bands with non-specific antibody binding. (d) Impairment of 9-cis retinoic acid (RA)-induced Rxrb-mediated transcription due to the absence of Cnot7. Wild-type and Cnot7−/− MEFs were transfected with Rxrb expression plasmid together with RXRE-luc reporter plasmid. Cells were incubated in the absence or presence of 9-cis retinoic acid (1 μM). Error bars represent s.d. Asterisk indicates a statistically significant difference between wild-type and Cnot7−/− MEFs (P < 0.01). Statistical differences between groups were assessed by Student's t-test. (e) Effect of Cnot7 on Rxrb DNA-binding. Increasing amounts of nuclear extracts (1 μg, +; 3 μg, ++; 9 μg, +++) isolated from testes of wild-type (lanes 2–4) or Cnot7−/− (lanes 5–7) mice were incubated with a fixed amount of radioactively labeled response element for EMSA. The DNA-binding activity of Rxrb from testes of wild-type mice (lane 8) was compared with that of testes from Cnot7−/− mice (lane 9) by supershift experiments with antibody to Rxrb. Levels of Rxrb expression were similar in testes from wild-type and Cnot7−/− mice (data not shown). The quantity of nuclear extracts used was confirmed with the oligonucleotide probe containing κB site from the mouse κ-light chain enhancer (lanes 10 and 11). (f) Functional associations between Cnot7 and Rxrb. Recombinant CNOT7 was produced in E. coli and added to the nuclear extracts of testes from Cnot7−/− mice before EMSA (lane 4, right panel). The left panel shows Coomassie brilliant blue staining of the recombinant CNOT7 protein.
We then examined whether Rxrb functions are affected in the absence of Cnot7. To measure the Rxrb-mediated transcription in the Cnot7−/− condition, we used primary mouse embryonic fibroblasts (MEFs). We transfected an Rxrb expression vector and a luciferase reporter plasmid containing an RXR response element (RXRE)-coupled thymidine kinase promoter into wild-type or Cnot7−/− MEFs. Luciferase reporter assays showed that Rxrb-mediated transcription in response to 9-cis retinoic acid was much lower in the absence of Cnot7 (Fig. 4d). In addition, electrophoretic mobility shift assays (EMSAs) showed that proteins from testes of Cnot7−/− mice bound to the RXRE less efficiently than those from testes of Cnot7+/+ mice (Fig. 4e). Reintroduction of recombinant CNOT7 in the nuclear extracts of testes of Cnot7−/− mice resulted in substantial recovery of the ability to bind the RXRE (Fig. 4f). Taken together, these findings suggest that the AF-1 domain is responsible for the Rxrb-Cnot7 interaction and that Cnot7 contributes to Rxrb function.
In this study, we found in vivo evidence that abnormalities during spermatogenesis in testes of Cnot7−/− mice are possibly caused by defects in the testicular somatic cells rather than in the germ cells. The function of testicular somatic cells is directed by pituitary gonadotropins secreted from the hypothalamic-pituitary axis. Among the gonadotropins, luteinizing hormone mainly stimulates testosterone production in the Leydig cells. Because serum testosterone level did not differ between male Cnot7+/− and Cnot7−/− mice (data not shown), hormonal regulation mediated through the hypothalamic-pituitary axis and Leydig cells seems to be little affected by the absence of Cnot7. The testicular phenotypes in Cnot7−/− mice seem to be caused by the functional defect of Sertoli cells.
Although the RXR family members Rxra, Rxrb and Rxrg share structural similarities, genetic analyses suggest that each has distinct roles in mice24,25,26. Our data show that testicular phenotypes of male Cnot7−/− mice are similar to those of Rxrb−/− mice (vacuole formation, failure in alignment of late spermatids, multiple generations of spermatids, lipid accumulation in the cytoplasm of Sertoli cells and no apparent phenotypic abnormalities in other adult tissues), suggesting that Cnot7 functionally interacts with Rxrb but not with other family members. Indeed, our data show that Rxrb interacts physically and functionally with CNOT7 through its AF-1 domain. Therefore, Cnot7 may function as a specific coregulator of Rxrb to contribute to spermatogenesis.
Little is known about the molecular mechanism of human infertility. It may be important to screen men with oligo-astheno-teratozoospermia for polymorphisms or mutations in CNOT7 to assess whether CNOT7 has a role in human infertility.
Methods
Generation of Cnot7−/− mice.
We subcloned the 18-kb genomic DNA fragment of Cnot7 into pBluescript. We replaced an exon of Cnot7 that contains the first methionine with lacZ and a pMC1neo poly(A) fragment flanked by loxP sites to be inserted in-frame with the first methionine to generate the targeting vector. The DT-A fragment was ligated at the 3′ end of the targeting vector for negative selection. We electroporated J1 embryonic stem cells with linearized targeting vector and subjected them to neomycin selection. We identified Cnot7 targeted clones by Southern-blot hybridization with the probe shown in Figure 1a, injected these cells into C57BL/6J blastocysts and mated chimeric offspring with C57BL/6J mice. We intercrossed heterozygous F1 mice to produce homozygous Cnot7−/− mice. We carried out all experiments with animals following guidelines for animal use issued by the Committee of Animal Experiments, Institute of Medical Science, University of Tokyo.
Conventional transmission electron microscopy.
We fixed adult testes with 2% glutaraldehyde in 0.2 M cacodylate buffer. After washing them in the same buffer, we cut the tissues into small pieces, immersed them in the same fixative for 2 h at 4 °C, rinsed them and fixed them with OsO4. The samples were dehydrated through graded ethanol and then embedded in Epon 812. We cut ultrathin sections on an ultramicrotome (model Ultracut E, Reihert-Jung) and stained them with uranyl acetate and lead citrate. We observed them with a JEOL 1200 EX (JEOL) transmission electron microscope.
IVF and ICSI.
We collected mature oocytes from the oviducts of female B6D2F1 mice that had been superovulated by the injection of 5 IU of equine chorionic gonadotrophin followed 48 h later by 5 IU of human chorionic gonadotropin. We carried out zona-free IVF using epididymal spermatozoa as described27. We fixed, stained and examined oocytes by phase-contrast microscopy 2 h after insemination. Oocytes with decondensed sperm nuclei were considered to be fertilized. For ICSI, we collected spermatogenic cells from the seminiferous tubules by a mechanical method and directly injected elongated spermatids at steps 9–11 into oocytes using a Piezo-driven micromanipulator28. Oocytes that survived injection were cultured in potassium simplex optimized medium and those developing to the 2-cell stage were transferred into the oviducts of pseudopregnant ICR females. We obtained live offspring at term (day 19.5) by Caesarian section or after natural delivery.
Testes histomorphometry.
We fixed testes in Bouin or 10% formalin neutral buffer solution, embedded them in paraffin and cut 7-mm sections. We dewaxed and stained sections with hematoxylin and eosin, with toluidine blue or by PAS reaction with standard procedures.
Analysis of apoptotic cells.
We detected apoptotic cells in sections of mouse testes in situ by TUNEL assay with an ApopTag peroxidase kit (Intergen). We counterstained sections with diluted hematoxylin.
Production of recipient mice and spermatogonial stem cell transplantation.
We obtained male WBB6F1-W/Wv mice from Japan SLC. We injected 6-week-old BALB/cA Jcl-nu males (CLEA Japan) intraperitoneally with freshly prepared busulfan (44 mg per kg body weight). Busulfan-treated mice were devoid of endogeneous spermatogenesis at the time of transplantation (∼6 weeks after busulfan treatment). We irradiated (12 Gy) the lower halves of the bodies of 14- to 18-d-old C57BL/6N mouse pups (CLEA Japan) to eliminate endogenous germ cells in the testes and used these mice for transplantation experiments 2 d later29. We prepared donor cell suspension (2–3 × 107 cells ml−1) from 8- to 10-week-old Cnot7−/− mice by a two-step enzymatic digestion technique16 and introduced ∼10 μl into the seminiferous tubules of each recipient mouse. We identified donor-derived areas of spermatogenesis in testes of recipients by staining with 5-bromo-4-chloro-3-indolyl β-D-galactoside (X-gal).
Cell culture.
We obtained MEFs from 14.5-d-old embryos by an established procedure14. We maintained MEFs, COS7 and TTE3 cells in Dulbecco's modified Eagle medium containing 10% fetal bovine serum and antibiotics.
Plasmids.
We amplified cDNA fragments for various portions of GST-fused Rxrb by PCR. The AF-1 domain of Rxrb was described previously30. Primer sequences for Rxrb mutants are available on request.
GST pull-down.
For GST pull-down assays, we lysed cells with RIPA buffer as described9 36 h after transfection. We purified GST fusion proteins with glutathione-Sepharose beads, resolved the bound proteins by SDS-PAGE and analyzed them by immunoblotting. Antibodies used for blotting were monoclonal antibody to FLAG (Sigma) and monoclonal antibody to GST (Santa Cruz Biotechnology).
Immunoprecipitation and immunoblotting.
We solubilized TTE3 cells in Triton X-100 lysis buffer (0.5% Triton X-100, 50 mM Tris (pH 7.4), 10% glycerol, 100 mM NaCl, 2 mM MgCl2, 0.1 mM CaCl2, 1 μM 9-cis retinoic acid and 25 μM MG132) supplemented with protease inhibitor cocktails (Sigma). We incubated precleared lysates sequentially with polyclonal antibodies to Cnot7 and protein A-Sepharose (Amersham Biosciences). We washed the immunoprecipitates at least four times with lysis buffer. We raised rabbit polyclonal antibodies against Cnot7 using a keyhole limpet hemocyanin–conjugated synthetic peptide with the sequence SYVQNGTGNAYEEEANKQS as immunogen and affinity-purified it. We used a monoclonal antibody to Rxrb for blotting, which was a gift from Perseus Proteomics.
Transactivation assay.
For Rxrb-mediated transactivation assay, we cotransfected MEFs (5 × 104 cells per well in 12-well tissue culture plates) with Lipofectamine (Invitrogen) and the following plasmids: (i) pSG5-Rxrb (0.2 μg), (ii) pGL3-RXRE-Luc (reporter plasmid; 0.05 μg) and (iii) pRL-TK (0.025 μg). We analyzed cell extracts for luciferase activity with a Dual-Luciferase Reporter System (Promega). Transfection efficiency was standardized with an internal control plasmid, pRL-TK. Data are shown as the average ± s.d. of three independent experiments, each done in triplicate.
Preparation of nuclear extracts and EMSA.
We isolated testes from 10- to 16-week-old Cnot7+/+ or Cnot7−/− mice, washed them in ice-cold phosphate-buffered saline and suspended them in hypotonic buffer (10 mM HEPES buffer (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol (DTT) and 0.2 mM phenylmethylsulfonyl fluoride (PMSF)). We broke cells with ten strokes with a type A Dounce homogenizer and two gentle strokes with a type B Dounce homogenizer. We collected nuclei by centrifugation for 10 min at 600g and 4 °C and resuspended them in 500 μl of hypotonic buffer and added 250 μl of low salt buffer (20 mM HEPES buffer (pH 7.9), 25% glycerol, 1.5 mM MgCl2, 20 mM KCl, 0.2 mM EDTA, 0.5 mM DTT and 0.5 mM PMSF). We then added 250 μl of high salt buffer (20 mM HEPES buffer (pH 7.9), 25% glycerol, 1.5 mM MgCl2, 1.2 M KCl, 0.2 mM EDTA, 0.5 mM DTT and 0.5 mM PMSF) dropwise. We extracted nuclei for 30 min at 4 °C with constant rotation, centrifuged the suspension for 30 min at 150,000g and 4 °C, collected the supernatant and used it for EMSAs. We used an RXRE composed of two half sites oriented as direct repeats with a 1-kb spacer element as the oligonucleotide probe. We incubated the nuclear extracts at room temperature for 20–30 min with the radioactively labeled oligonucleotide probe (40,000–60,000 c.p.m.) in 15 μl of binding buffer (15 mM Tris-HCl (pH 7.5), 75 mM NaCl, 1.5 mM EDTA, 15 mM DTT, 7.5% glycerol, 1 μg μl−1 bovine serum albumin and 0.3% Nonidet P-40). We then resolved the resulting DNA-protein complexes by nondenaturing gel electrophoresis and visualized them by autoradiography. For supershift experiments, we used antibody to Rxrb (Santa Cruz Biotechnology; sc-831).
Protein cloning, expression, and purification.
We cloned a DNA fragment encoding the full-length human CNOT7 protein into pGEX-6P-3 (Amersham Biosciences). We expressed the protein in Escherichia coli BL21, purified it by affinity chromatography on glutathione Sepharose 4B and cleaved it from GST with PreScission protease (Amersham Biosciences). The protein samples from vector-transfected E. coli were similarly treated (Vehicle).
Sperm count and motility analysis.
We placed cauda epididymides in 0.1 ml of motile buffer (120 mM NaCl, 5 mM KCl, 25 mM NaHCO3, 1.2 mM KH2PO4, 1.2 mM MgSO4 and 1.3 mM CaCl2). We minced the tissue with scissors and incubated it at 37 °C for 5 min to allow sperm dispersal.
References
Matzuk, M.M. & Lamb, D.J. Genetic dissection of mammalian fertility pathways. Nat. Cell Biol. 4 Suppl, S41– S49 (2002).
Prevot, D. et al. Relationships of the antiproliferative proteins BTG1 and BTG2 with CAF1, the human homolog of a component of the yeast CCR4 transcriptional complex: involvement in estrogen receptor alpha signaling pathway. J. Biol. Chem. 276, 9640– 9648 (2001).
Kastner, P. et al. Abnormal spermatogenesis in RXRβ mutant mice. Genes Dev. 10, 80– 92 (1996).
Draper, M.P., Salvadore, C. & Denis, C.L. Identification of a mouse protein whose homolog in Saccharomyces cerevisiae is a component of the CCR4 transcriptional regulatory complex. Mol. Cell. Biol. 15, 3487– 3495 (1995).
Collart, M.A. Global control of gene expression in yeast by the Ccr4-Not complex. Gene 313, 1– 16 (2003).
Denis, C.L. & Chen, J. The CCR4-NOT complex plays diverse roles in mRNA metabolism. Prog. Nucleic Acid Res. Mol. Biol. 73, 221– 250 (2003).
Rouault, J.P. et al. Interaction of BTG1 and p53-regulated BTG2 gene products with mCaf1, the murine homolog of a component of the yeast CCR4 transcriptional regulatory complex. J. Biol. Chem. 273, 22563– 22569 (1998).
Albert, T.K. et al. Isolation and characterization of human orthologs of yeast CCR4-NOT complex subunits. Nucleic Acids Res. 28, 809– 817 (2000).
Ikematsu, N. et al. Tob2, a novel anti-proliferative Tob/BTG1 family member, associates with a component of the CCR4 transcriptional regulatory complex capable of binding cyclin-dependent kinases. Oncogene 18, 7432– 7441 (1999).
Yoshida, Y., Hosoda, E., Nakamura, T. & Yamamoto, T. Association of ANA, a member of the antiproliferative Tob family proteins, with a Caf1 component of the CCR4 transcriptional regulatory complex. Jpn. J. Cancer Res. 92, 592– 596 (2001).
Prevot, D. et al. The leukemia-associated protein Btg1 and the p53-regulated protein Btg2 interact with the homeoprotein Hoxb9 and enhance its transcriptional activation. J. Biol. Chem. 275, 147– 153 (2000).
Yoshida, Y. et al. Negative regulation of BMP/Smad signaling by Tob in osteoblasts. Cell 103, 1085– 1097 (2000).
Tzachanis, D. et al. Tob is a negative regulator of activation that is expressed in anergic and quiescent T cells. Nat. Immunol. 2, 1174– 1182 (2001).
Yoshida, Y. et al. Mice lacking a transcriptional corepressor Tob are predisposed to cancer. Genes Dev. 17, 1201– 1206 (2003).
Brinster, R.L. & Zimmermann, J.W. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. USA 91, 11298– 11302 (1994).
Ogawa, T., Dobrinski, I., Avarbock, M.R. & Brinster, R.L. Transplantation of male germ line stem cells restores fertility in infertile mice. Nat. Med. 6, 29– 34 (2000).
Ogawa, T., Ohmura, M., Yumura, Y., Sawada, H. & Kubota, Y. Expansion of murine spermatogonial stem cells through serial transplantation. Biol. Reprod. 68, 316– 322 (2003).
Lufkin, T. et al. High postnatal lethality and testis degeneration in retinoic acid receptor α mutant mice. Proc. Natl. Acad. Sci. USA 90, 7225– 7229 (1993).
Eddy, E.M. et al. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology 137, 4796– 4805 (1996).
Charest, N.J. et al. A frameshift mutation destabilizes androgen receptor messenger RNA in the Tfm mouse. Mol. Endocrinol. 5, 573– 581 (1991).
Livera, G., Rouiller-Fabre, V., Pairault, C., Levacher, C. & Habert, R. Regulation and perturbation of testicular functions by vitamin A. Reproduction 124, 173– 180 (2002).
Tabuchi, Y. et al. Development of the conditionally immortalized testicular Sertoli cell line TTE3 expressing Sertoli cell specific genes from mice transgenic for temperature sensitive simian virus 40 large T antigen gene. J. Urol. 167, 1538– 1545 (2002).
Mangelsdorf, D.J. et al. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 6, 329– 344 (1992).
Sucov, H.M. et al. RXRα mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes Dev. 8, 1007– 1018 (1994).
Kastner, P. et al. Genetic analysis of RXRα developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell 78, 987– 1003 (1994).
Krezel, W. et al. RXRγ null mice are apparently normal and compound RXRα+/−/RXRβ−/−/RXRγ−/− mutant mice are viable. Proc. Natl. Acad. Sci. USA 93, 9010– 9014 (1996).
Maleszewski, M., Kimura, Y. & Yanagimachi, R. Sperm membrane incorporation into oolemma contributes to the oolemma block to sperm penetration: evidence based on intracytoplasmic sperm injection experiments in the mouse. Mol. Reprod. Dev. 44, 256– 259 (1996).
Ogonuki, N. et al. Fertilization of oocytes and birth of normal pups following intracytoplasmic injection with spermatids in mastomys (Praomys coucha). Biol. Reprod. 68, 1821– 1827 (2003).
Creemers, L.B. et al. Transplantation of germ cells from glial cell line-derived neurotrophic factor-overexpressing mice to host testes depleted of endogenous spermatogenesis by fractionated irradiation. Biol. Reprod. 66, 1579– 1584 (2002).
Leid, M. et al. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell 68, 377– 395 (1992).
Acknowledgements
We thank J. Yanagisawa, Y. Sugitani, T. Nakazawa, M. Ohsugi, T. Tezuka, K. Semba and M. Noda for discussions; S. Kato for providing expression vectors for nuclear receptors and reporter plasmids containing response elements for nuclear receptors; N. Yanai for providing TTE3 cells; and H. Yamanaka, N. Kusaka, A. Nakamura, M. Yoneda, A. Moriya and F. Suzuki-Toyota for technical support. This work was supported by a Grant for Advanced Cancer Research from the Ministry of Education, Science, Sports, and Culture of Japan and grants from the Organization for Pharmaceutical Safety and Research of Japan, and from the Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Nakamura, T., Yao, R., Ogawa, T. et al. Oligo-astheno-teratozoospermia in mice lacking Cnot7, a regulator of retinoid X receptor beta. Nat Genet 36, 528–533 (2004). https://doi.org/10.1038/ng1344
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1344
This article is cited by
-
ARRDC5 expression is conserved in mammalian testes and required for normal sperm morphogenesis
Nature Communications (2023)
-
The CCR4–NOT deadenylase complex safeguards thymic positive selection by down-regulating aberrant pro-apoptotic gene expression
Nature Communications (2020)
-
Testis Transcriptome Modulation in Klinefelter Patients with Hypospermatogenesis
Scientific Reports (2017)
-
The CCR4-NOT complex contributes to repression of Major Histocompatibility Complex class II transcription
Scientific Reports (2017)
-
CNOT3 suppression promotes necroptosis by stabilizing mRNAs for cell death-inducing proteins
Scientific Reports (2015)