Abstract
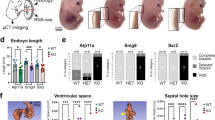
In marsupials and mice, the paternally derived X chromosome is preferentially inactivated in the placental tissues of female embryos1,2,3,4. We show here that the X-linked homeobox gene Esx1 (Refs 5,6), whose expression is restricted to extraembryonic tissues, is a chromosomally imprinted regulator of placental morphogenesis and trophoblast differentiation. Heterozygous female mice that inherited a mutant Esx1 allele from their father developed normally. Heterozygous females that inherited the Esx1 mutation from their mother, however, were born 20% smaller than normal and are identical in phenotype to hemizygous mutant males and homozygous mutant females. Although Esx1 mutant embryos were initially comparable in size with controls at 13.5 days post coitum (dpc), their placentas were significantly larger. Defects in the morphogenesis of the labyrinthine layer were observed as early as 11.5 dpc. Subsequently, vascularization abnormalities developed at the maternal-fetal interface, causing fetal growth retardation. These results identify Esx1 as the first essential X-chromosome-imprinted regulator of placental development that influences fetal growth, and may aid our understanding human placental insufficiency syndromes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tagaki, N. & Sasaki, M. Preferential expression of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature 256, 640–642 (1975).
West, J.D., Frels, W.I., Chapman, V.M. & Papaioannou, V.E. Preferential expression of the maternally derived X chromosome in the mouse yolk sac. Cell 12, 873– 882 (1977).
Harper, M.I., Foster, M. & Monk, M. Preferential paternal X inactivation in extra-embryonic tissues of early mouse embryos. J. Embryol. Exp. Morphol. 67, 127–138 (1982).
VandeBerg, J.L., Robinson, E.S., Samollow, P.B. & Johnston, P. X-linked gene expression and X-chromosome inactivation: marsupials, mouse and man compared. in Isozymes: Current Topics in Biological and Medical Research, Vol. 15: Genetics, Development, and Evolution (Academic Press, San Diego, 1987).
Branford, W.W. et al. Spx1, a novel X-linked homeobox gene expressed during spermatogenesis. Mech. Dev. 65, 87–98 (1997).
Li, Y., Lemaire, P. & Behringer, R.R. Esx1, a novel X chromosome-linked homeobox gene expressed in mouse extraembryonic tissues and male germ cells. Dev. Biol. 188, 85–95 (1997).
Hernandez-Verdun, D. Morphogenesis of the syncytium in the mouse placenta. Cell Tiss. Res. 148, 381–396 (1974).
Kaufman, M.H. Atlas of Mouse Development (Academic Press, San Diego, 1992).
Jollie, W.P. Fine structural changes in placental labyrinthine of the rat with increasing gestational age. J. Ultrastruct. Res. 10, 27–47 (1964).
Davies, J. & Glasser, S.R. Histological and fine structural observations on the placenta of the rat. Acta Anat. 69, 542–608 (1968).
Lescisin, K.R., Varmuza, S. & Rossant, J. Isolation and characterization of a novel trophoblast-specific cDNA in the mouse. Genes Dev. 2, 1639– 1646 (1988).
Cross, J.C., Werb, Z. & Fisher, S. Implantation and the placenta: key pieces of the development puzzle. Science 266, 1508– 1518 (1994).
Muller, H.K. et al. Ultraviolet irradiation of murine skin alters cluster formation between lymph node dendritic cells and specific T lymphocytes. Cell. Immunol. 157, 263–276 (1994).
Harbers, K. et al. Provirus integration into a gene encoding a ubiquitin-conjugating enzyme results in a placental defect and embryonic lethality. Proc. Natl Acad. Sci. USA 93, 12412– 12417 (1996).
Loke, Y.W. & King, A. Human Implantation (Cambridge University Press, Cambridge, UK, 1995).
Barker, D.J. et al. Fetal nutrition and cardiovascular disease in adult life. Lancet 341, 938–941 (1993).
Mansour, S.L., Thomas, K.R. & Capecchi, M.R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature 336, 348– 352 (1988).
McMahon, A.P. & Bradley, A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell 62, 1073–1085 (1990).
Behringer, R.R., Finegold, M.J. & Cate, R.L. Mullerian-inhibiting substance function during mammalian sexual development. Cell 79, 415– 425 (1994).
Uwanogho, D. et al. Embryonic expression of chicken Sox2, Sox3 and Sox11 genes suggested an interactive role in neuronal development. Mech. Dev. 49, 23–36.
Acknowledgements
We thank A. Bradley for the AB-1 ES and SNL 76/7 STO cell lines, A. Nagy for the ploxPneo-1 plasmid, K. Mahon for probes, K. Dunner Jr and C. Bucana for TEM, and S. Glasser, R. Farnsworth and K. Mahon for helpful discussions. The M.D. Anderson Cancer Center High Resolution Electron Microscopy Facility is supported by an institutional core grant from the National Institutes of Health (NIH). This study was supported by NIH grant HD30284 to R.R.B.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Behringer, R. Esx1 is an X-chromosome-imprinted regulator of placental development and fetal growth. Nat Genet 20, 309–311 (1998). https://doi.org/10.1038/3129
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/3129
This article is cited by
-
X-linked genes influence various complex traits in dairy cattle
BMC Genomics (2023)
-
Conflict and the evolution of viviparity in vertebrates
Behavioral Ecology and Sociobiology (2022)
-
Association of ESX1 gene variants with non-obstructive azoospermia in Chinese males
Scientific Reports (2021)
-
Abnormal gene expression in regular and aggregated somatic cell nuclear transfer placentas
BMC Biotechnology (2017)
-
Sonic hedgehog through Gli2 and Gli3 is required for the proper development of placental labyrinth
Cell Death & Disease (2015)