Abstract
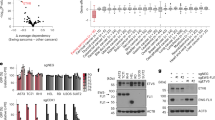
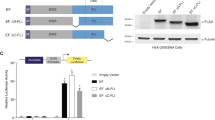
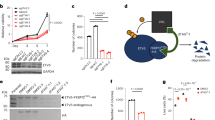
Chromosomal translocations resulting in the expression of chimaeric transcription factors are frequently observed in tumour cells1, and have been suggested to be a common mechanism in human carcinogenesis. Ewing sarcoma and related peripheral primitive neuroectodermal tumours share recurrent translocations that fuse the gene EWSR1 (formerly EWS) from 22q–12 to FLI1 and genes encoding other ETS transcription factors2,3,4 (which bind DNA through the conserved ETS domain5,6). It has been shown that transduction of the gene EWSR1-FLI1 (encoding EWS-FLI1 protein) can transform NIH3T3 cells, and that mutants containing a deletion in either the EWS domain or the DNA-binding domain in FLI1 lose this ability5,6,7,8. This indicates that the EWS-FLI1 fusion protein may act as an aberrant transcription factor, but the exact mechanism of oncogenesis remains unknown. Because ETS transcription factors regulate expression of TGFBR2 (encoding the TGF-β type II receptor, TGF-β RII; Refs 9,14), a putative tumour suppressor gene, we hypothesized that TGFBR2 may be a target of the EWS-FLI1 fusion protein. We show here that embryonic stem (ES) cell lines with the EWSR1-FLI1 fusion have reduced TGF-β sensitivity, and that fusion-positive ES cells and primary tumours both express low or undetectable levels of TGFBR2 mRNA and protein product. Co-transfection of FLI1 and the TGFBR2 promoter induces promoter activity, whereas EWSR1-FLI1 leads to suppression of TGFBR2 promoter activity and FLI1-induced promoter activity. Introduction of EWSR1-FLI1 into cells lacking the EWSR1-FLI1 fusion suppresses TGF-β RII expression, whereas antisense to EWSR1-FLI1 in ES cell lines positive for this gene fusion restores TGF-β RII expression. Furthermore, introduction of normal TGF-β RII into ES cell lines restores TGF-β sensitivity and blocks tumorigenicity. Our results implicate TGF-β RII as a direct target of EWS-FLI1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rabbitts, T.H. Chromosomal translocations in human cancer. Nature 372, 143–149 (1994).
Delattre, O. et al. Gene fusion with an ETS DNA binding domain caused by chromosome translocation in human cancers. Nature 359, 162–165 (1992).
Sorensen, P.H.B. et al. A second Ewing's sarcoma translocation, t(21;22), fuses the EWS gene to another ETS-family transcription factor, ERG. Nature Genet. 6, 146–151 (1994).
Jeon, I.-S. et al. A variant Ewing's sarcoma translocation (7;22) fuses the EWS gene to the ETS gene ETV1. Oncogene 10, 1229–1234 (1995).
May, W. et al. Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc. Natl Acad. Sci. USA 90, 5752–5756 (1993).
Bailly, R.-A. et al. DNA-binding and transcriptional activation properties of the EWS-FLI-1 fusion protein resulting from the t(11;22) translocation in Ewing sarcoma. Mol. Cell. Biol. 14, 3230–3241 (1994).
Braun, B.S., Frieden, R., Lessnick, S.L., May, W.A. & Denny, C.T. Identification of target genes for the Ewing's sarcoma EWS-FLI1 fusion protein by representational difference analysis. Mol. Cell. Biol. 15, 4623–4630 (1995).
May, W.A. et al. EWS/FLI1-induced manic fringe renders NIH-3T3 cells tumourigenic. Nature Genet. 17, 495–497 (1997).
Choi, S.-G. et al. A novel ets-related transcription factor, ERT/ESX/ESE-1, regulates expression of the transforming growth factor-β type II receptor. J. Biol. Chem. 273, 110–117 (1998).
Chang, C.-H. et al. ESX: a structurally unique Ets overexpressed early during human breast tumourigenesis. Oncogene 14, 1617–1622 (1997).
Oettgen, P. et al. Isolation and characterization of a novel epithelial specific transcription factor, ESE-1, a member of the ets family. Mol. Cell. Biol. 17, 4419–4433 (1997).
Tymms, M.J. et al. A novel epithelial-expressed ETS gene, ELF3: human and murine cDNA sequences, murine genomic organization, human mapping to 1q32.2 and expression in tissues and cancer. Oncogene 15, 2449–2462 (1997).
Andreoli, J.M. et al. The expression of a novel, epithelium-specific ets transcription factor is restricted to the most differentiated layers in the epidermis. Nucleic Acids Res. 25, 4287–4295 (1997).
Bae, H.W. et al. Characterization of the promoter region of the human transforming growth factor-β type II receptor gene. J. Biol. Chem. 270, 29460–29468 (1995).
Zawel, L. et al. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol. Cell 1, 611–617 (1998).
De Alava, E. et al. EWS/FLI1 fusion transcript structure is an independent determinant of progrosis in Ewing's sarcoma. J. Clin. Oncol 16, 1248–1255 (1998).
Thompson, A.D. et al. EAT-2 is a novel SH2 domain containing protein that is up regulated by Ewing's sarcoma EWS/FLI1 fusion gene. Oncogene 15, 2649–2658 (1996).
Arvand, A. et al. EWS/FLI1 up regulates mE2-C, a cyclin-selective ubiquitin conjugating enzyme involved in cyclin B destruction. Oncogene 17, 2039–2045.
Chang, J. et al. Expression of TGF-β type II receptor reduces tumourigenicity in human gastric cancer cells. Cancer Res. 57, 1–4 (1998).
Derynck, R. et al. Human transforming growth factor-β cDNA sequence and expression in tumour cell lines. Nature 316, 701–705 (1985).
Acknowledgements
We thank D. Wilson and S.J. Baker for FLI1 and EWSR1-FLI1 expression vectors; S.E. Kern for the SBE4-Luc construct; and A.B. Roberts, T. Parks and J. Letterio for discussion and critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hahm, KB., Cho, K., Lee, C. et al. Repression of the gene encoding the TGF-β type II receptor is a major target of the EWS-FLI1 oncoprotein. Nat Genet 23, 222–227 (1999). https://doi.org/10.1038/13854
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/13854
This article is cited by
-
CRISPR activation screen identifies TGFβ-associated PEG10 as a crucial tumor suppressor in Ewing sarcoma
Scientific Reports (2022)
-
TrkC, a novel prognostic marker, induces and maintains cell survival and metastatic dissemination of Ewing sarcoma by inhibiting EWSR1-FLI1 degradation
Cell Death & Disease (2022)
-
The Tumor Microenvironment of Pediatric Sarcoma: Mesenchymal Mechanisms Regulating Cell Migration and Metastasis
Current Oncology Reports (2019)
-
Ewing sarcoma
Nature Reviews Disease Primers (2018)
-
EWS-FLI1-mediated suppression of the RAS-antagonist Sprouty 1 (SPRY1) confers aggressiveness to Ewing sarcoma
Oncogene (2017)