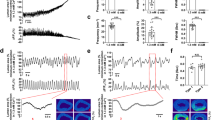
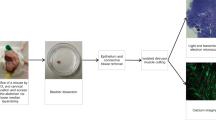
Abstract
Scientists interested in the smooth muscles of the urinary tract, and their control, have recently been studying cells in the interstitium of tissues that express the c-kit antigen (Kit+ cells). These cells have morphologic features that are reminiscent of the well-described pacemaker cells in the gut, the interstitial cells of Cajal (ICC). The spontaneous contractile behavior of muscles in the urinary tract varies widely, and it is clear that urinary tract Kit+ interstitial cells cannot be playing an identical role to that played by the ICC in the gut. Nevertheless, there is increasing evidence that they do play a role in modulating the contractile behavior of adjacent smooth muscle, and might also be involved in mediating neural control. This review outlines the properties of ICC in the gut, and gives an account of the discovery of cells in the interstitium of the main components of the urinary tract. The physiologic properties of such cells and the functional implications of their presence are discussed, with particular reference to the bladder. In this organ, Kit+ cells are found under the lamina propria, where they might interact with the urothelium and with sensory nerves, and also between and within the smooth-muscle bundles. Confocal microscopy and calcium imaging are being used to assess the physiology of ICC and their interactions with smooth muscles. Differences in the numbers of ICC are seen in smooth muscle specimens obtained from patients with various pathologies; in particular, bladder overactivity is associated with increased numbers of these cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cajal SR (1893) Sur les ganglions et plexus nerveux de l'intestin. C R Seances Soc Biol Fil 45: 217–223
Cajal SR (1911) Histologie du Système Nerveux de l'Homme et des Vertébrés. Paris: Maloine
Thuneberg L (1999) One hundred years of interstitial cells of Cajal. Microsc Res Tech 47: 223–238
Sanders KM (1996) A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology 111: 492–515
Rumessen JJ and Thuneberg L (1996) Pacemaker cells in the gastrointestinal tract: interstitial cells of Cajal. Scand J Gastroenterol 216 (Suppl): 82–94
Cousins HM et al. (2003) Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J Physiol 550: 829–844
Ward SM and Sanders KM (2001) Interstitial cells of Cajal: primary targets of enteric motor innervation. Anat Rec 262: 125–135
Horowitz B et al. (1999) Cellular and molecular basis for electrical rhythmicity in gastrointestinal muscles. Annu Rev Physiol 61: 19–43
Komuro T et al. (1999) Ultrastructural characterization of the interstitial cells of Cajal. Arch Histol Cytol 62: 295–316
Ward SM et al. (2003) Propagation of slow waves requires IP3 receptors and mitochondrial Ca2+ uptake in canine colonic muscles. J Physiol 549: 207–218
Koh SD et al. (2003) Conductances responsible for slow wave generation and propagation in interstitial cells of Cajal. Curr Opin Pharmacol 3: 579–582
Mikkelsen HB et al. (1998) Action potential generation, Kit receptor immunohistochemistry and morphology of steel-Dickie (Sl/Sld) mutant mouse small intestine. Neurogastroenterol Motil 10: 11–26
Sanders KM et al. (2002) Physiology and pathophysiology of the interstitial cells of Cajal: from bench to bedside. IV. Genetic and animal models of GI motility disorders caused by loss of interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol 282: 747–756
Smet PJ et al. (1996) Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience 71: 337–348
Sergeant GP et al. (2000) Specialised pacemaking cells in the rabbit urethra. J Physiol 526: 359–366
Sergeant GP et al. (2001) Spontaneous Ca2+ activated Cl− currents in isolated urethral smooth muscle cells. J Urol 166: 1161–1166
Hollywood MA et al. (2003) Activation of Ca2+-activated Cl− current by depolarizing steps in rabbit urethral interstitial cells. Am J Physiol Cell Physiol 285: 327–333
Johnston L et al. (2005) Calcium oscillations in interstitial cells of the rabbit urethra. J Physiol 565: 449–461
McCloskey KD and Gurney AM . (2002) Kit positive cells in the guinea pig bladder. J Urol 168: 832–836
Davidson RA and McCloskey KD (2005) Morphology and localization of interstitial cells in the guinea-pig bladder: structural relationships with smooth muscle and neurons. J Urol 173: 1385–1390
Hashitani H et al. (2004) Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol 559: 567–581
Sui GP et al. (2002) Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int 90: 118–129
Gosling JA and Dixon JS (1972) Structural evidence in support of an urinary tract pacemaker. Br J Urol 44: 550–560
Klemm MF et al. (1999) Identification of the cells underlying pacemaker activity in the guinea-pig upper urinary tract. J Physiol 519: 867–884
Pezzone MA et al. (2003) Identification of c-kit-positive cells in the mouse ureter: the interstitial cells of Cajal of the urinary tract. Am J Physiol Renal Physiol 284: 925–929
Metzger R et al. (2004) Cajal-like cells in the human upper urinary tract. J Urol 172: 769–772
Solari V et al. (2003) Altered expression of interstitial cells of Cajal in congenital ureteropelvic junction obstruction. J Urol 170: 2420–2422
Exintaris B et al. (2002) Spontaneous slow wave and contractile activity of the guinea pig prostate. J Urol 168: 315–322
Van der Aa F et al. (2003) Interstitial cells in the human prostate: a new therapeutic target? Prostate 56: 250–255
Hashitani H and Suzuki H (2004) Identification of interstitial cells of Cajal in corporal tissues of the guinea-pig penis. Br J Pharmacol 141: 199–204
Burton LD et al. (2000) P2X2 receptor expression by interstitial cells of Cajal in vas deferens implicated in semen emission. Auton Neurosci 84: 147–161
Drake MJ et al. (2003) Morphology, phenotype and ultrastructure of fibroblastic cells from normal and neuropathic human detrusor: absence of myofibroblast characteristics. J Urol 169: 1573–1576
Wu C et al. (2004) Purinergic regulation of guinea pig suburothelial myofibroblasts. J Physiol 559: 231–243
Wiseman OJ et al. (2003) The role of the human bladder lamina propria myofibroblast. BJU Int 91: 89–93
Powell DW et al. (1999) Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol 277: 1–9
Hashitani H et al. (1996) Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br J Pharmacol 118: 1627–1632
Cotton KD et al. (1997) Ca2+ current and Ca2+-activated chloride current in isolated smooth muscle cells of the sheep urethra. J Physiol 505: 121–131
Turner WH and Brading AF (1997) Smooth muscle of the bladder in the normal and the diseased state: pathophysiology, diagnosis and treatment. Pharmacol Ther 75: 77–110
Daniel EE et al. (1983) Structural bases for neural and myogenic control of human detrusor muscle. Can J Physiol Pharmacol 61: 1247–1273
Bramich NJ and Brading AF (1996) Electrical properties of smooth muscle in the guinea-pig urinary bladder. J Physiol 492: 185–198
Hanani M and Brading AF (2000) Electrical coupling in smooth muscles. Is it universal? J Basic Clin Physiol Pharmacol 11: 321–330
Hashitani H and Brading AF (2003) Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol 140: 146–158
Hashitani H and Brading AF (2003) Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol 140: 159–169
Hashitani H et al. (2001) Origin and propagation of spontaneous excitation in smooth muscle of the guinea-pig urinary bladder. J Physiol 530: 273–286
Kubota Y et al. (2004) Investigation of the effect of the c-kit inhibitor Glivec on isolated guinea-pig detrusor preparations. Auton Neurosci 115: 64–73
Biers SM (2005) Novel pharmological treatments for detrusor overactivity [thesis]. Oxford: Oxford University.
Gillespie JI (2004) The autonomous bladder: a view of the origin of bladder overactivity and sensory urge. BJU Int 93: 478–483
De Ridder D et al. (1999) Nitric oxide synthase expression in neurogenic bladder disease: a pilot study. Acta Neurol Belg 99: 57–60
Moon A (2002) Influence of nitric oxide signalling pathways on pre-contracted human detrusor smooth muscle in vitro. BJU Int 89: 942–949
Ost D et al. (2002) Topography of the vanilloid receptor in the human bladder: more than just the nerve fibers. J Urol 168: 293–297
Fry CH et al. (2005) Cell biology. In Incontinence: 3rd International Consultation on Incontinence, 313–362 (Eds Abrams P et al.). Plymouth: Health Publication Ltd
Kubota Y et al.: The effects of imatinib mesylate (Glivec) as a c-kit tyrosine kinase inhibitor in the guinea-pig urinary bladder. Neurourol Urodyn, in press
Piotrowska AP et al. (2004) Interstitial cells of Cajal in the human normal urinary bladder and in the bladder of patients with megacystis-microcolon intestinal hypoperistalsis syndrome. BJU Int 94: 143–146
Acknowledgements
The authors would like to thank Professor Giorgio Gabella for helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Brading, A., McCloskey, K. Mechanisms of Disease: specialized interstitial cells of the urinary tract—an assessment of current knowledge. Nat Rev Urol 2, 546–554 (2005). https://doi.org/10.1038/ncpuro0340
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpuro0340
This article is cited by
-
Effect of acupuncture at points selected from different regions on SCF-kit signaling pathway in diabetic gastroparesis rats
Journal of Acupuncture and Tuina Science (2017)
-
Effect of anticholinergics on the overactive bladder and bowel domain of the electronic personal assessment questionnaire (ePAQ)
International Urogynecology Journal (2015)
-
COX Inhibitors and Overactive Bladder: The Potential for Future Therapy
Current Bladder Dysfunction Reports (2010)