Abstract
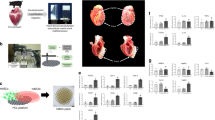
Autologous skeletal myoblast (ASM) transplantation is being explored as a possible therapy for patients who have suffered a myocardial infarction. Our initial experience with direct injection during coronary artery bypass grafting demonstrated that this method of delivery was both feasible and safe. In addition, proof of concept of the engraftment and survival of ASMs was shown. However, since many patients who have survived a myocardial infarction are not candidates for surgery, a less invasive delivery method is preferred. We implemented a series of translational research steps to bring catheter-based technology to a clinical application. This included assessing the biocompatibility of the ASM and a novel needle injection catheter using a 3-dimensional endoventricular navigation system, the bioretention and biodistribution of ASMs in a porcine model of myocardial infarction, and the safety and efficacy of ASM transplantation for cardiac function in the porcine model. After catheter functionality had been demonstrated, electromechanical mapping was used to assess the viability in the region of ASM transplantation, and echocardiography, electrocardiogram, and angiography tests were used to assess cardiac function 2 months after ASM transplantation. The results from these preclinical studies were used as a foundation for application of these concepts to a human clinical trial. Here we review the results from our preclinical experiments and surgical delivery clinical trial, and describe the recent clinical studies undertaken to assess the safety and feasibility of catheter-based ASM transplantation into human subjects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
American Heart Association (2004) Heart disease and stroke statistics—2004 update. [http://www.americanheart.org/downloadable/heart/HS_State_02.pdf] (accessed 26 October 2005)
Beltrami AP et al. (2003) Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114: 763–776
Mauro A (1961) Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9: 493–495
Dib N et al. (2005) Feasibility and safety of autologous myoblast transplantation in patients with ischemic cardiomyopathy. Cell Transplant 14: 11–19
Dib N et al. (2005) Safety and feasibility of autologous myoblast transplantation in patients with ischemic cardiomyopathy: four-year follow-up. Circulation 112: 1748–1755
Pagani FD et al. (2003) Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol 41: 879–888
Menasché P et al. (2003) Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol 41: 1078–1083
Herreros J et al. (2003) Autologous intramyocardial injection of cultured skeletal muscle-derived stem cells in patients with non-acute myocardial infarction. Eur Heart J 24: 2012–2020
Oron U et al. (2000) Technical delivery of myogenic cells through an endocardial injection catheter for myocardial cell implantation. Int J Cardiovasc Intervent 3: 227–230
Dib N et al.: A percutaneous swine model of myocardial infarction. J Pharmacol Toxicol Methods, in press
Kornowski R et al. (1998) Preliminary animal and clinical experiences using an electromechanical endocardial mapping procedure to distinguish infarcted from healthy myocardium. Circulation 98: 1116–1124
Koch KC et al. (2001) Myocardial viability assessment by endocardial electroanatomic mapping: comparison with metabolic imaging and functional recovery after coronary revascularization. J Am Coll Cardiol 38: 91–98
Criteria Committee of the New York Heart Association (1994) Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels, edn 9, 253–256. Boston: Little, Brown
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Opie, S., Dib, N. Surgical and catheter delivery of autologous myoblasts in patients with congestive heart failure. Nat Rev Cardiol 3 (Suppl 1), S42–S45 (2006). https://doi.org/10.1038/ncpcardio0399
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio0399
This article is cited by
-
Regenerative Medicine Approaches in Bioengineering Female Reproductive Tissues
Reproductive Sciences (2021)
-
Intramyocardial Navigation and Mapping for Stem Cell Delivery
Journal of Cardiovascular Translational Research (2010)
-
Science and engineering of electrospun nanofibers for advances in clean energy, water filtration, and regenerative medicine
Journal of Materials Science (2010)
-
Catheter-based delivery of cells to the heart
Nature Clinical Practice Cardiovascular Medicine (2006)