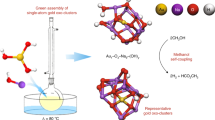
Abstract
Selective coupling of oxygenates is critical to many synthetic processes, including those necessary for the development of alternative fuels. We report a general process for selective coupling of aldehydes and methanol as a route to ester synthesis. All steps are mediated by oxygen-covered metallic gold nanoparticles on Au(111). Remarkably, cross-coupling of methanol with formaldehyde, acetaldehyde, benzaldehyde and benzeneacetaldehyde to methyl esters is promoted by oxygen-covered Au(111) below room temperature with high selectivity. The high selectivity is attributed to the ease of nucleophilic attack of the aldehydes by the methoxy intermediate—formed from methanol on the surface—which yields the methyl esters. The competing combustion occurs via attack of both methanol and the aldehydes by oxygen. The mechanistic model constructed in this study provides insight into factors that control selectivity and clearly elucidates the crucial role of Au nanoparticles as active species in the catalytic oxidation of alcohols, even in solution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Abad, A., Concepcion, P., Corma, A. & Garcia, H. A collaborative effect between gold and a support induces the selective oxidation of alcohols. Angew. Chem. Int. Ed. 44, 4066–4069 (2005).
Abad, A., Corma, A. & Garcia, H. Catalyst parameters determining activity and selectivity of supported gold nanoparticles for the aerobic oxidation of alcohols: The molecular reaction mechanism. Chem. Eur. J. 14, 212–222 (2008).
Biella, S. & Rossi, M. Gas phase oxidation of alcohols to aldehydes or ketones catalysed by supported gold. Chem. Commun. 378–379 (2003).
Carrettin, S. et al. Oxidation of glycerol using supported Pt, Pd and Au catalysts. Phys. Chem. Chem. Phys. 5, 1329–1336 (2003).
Enache, D. I., Knight, D. W. & Hutchings, G. J. Solvent-free oxidation of primary alcohols to aldehydes using supported gold catalysts. Catal. Lett. 103 (1–2), 43–52 (2005).
Gong, J. & Mullins, C. B. Selective oxidation of ethanol to acetaldehyde on gold. J. Am. Chem. Soc. 130, 16458–16459 (2008).
Hayashi, T., Inagaki, T., Itayama, N. & Baba, H. Selective oxidation of alcohol over supported gold catalysts: methyl glycolate formation from ethylene glycol and methanol. Catal. Today. 117, 210–213 (2006).
Hou, W. B., Dehm, N. A. & Scott, R. W. J. Alcohol oxidations in aqueous solutions using Au, Pd, and bimetallic AuPd nanoparticle catalysts. J. Catal. 253, 22–27 (2008).
Jorgensen, B., Christiansen, S. E., Thomsen, M. L. D. & Christensen, C. H. Aerobic oxidation of aqueous ethanol using heterogeneous gold catalysts: Efficient routes to acetic acid and ethyl acetate. J. Catal. 251, 332–337 (2007).
Su, F. Z. et al. Gold supported on nanocrystalline beta-Ga2O3 as a versatile bifunctional catalyst for facile oxidative transformation of alcohols, aldehydes, and acetals into esters. Chem. Eur. J. 14, 7131–7135 (2008).
Wang, L. C. et al. Solvent-free selective oxidation of alcohols by molecular oxygen over gold nanoparticles supported on beta-MnO2 nanorods. Appl. Catal. A 344, 150–157 (2008).
Wang, X. G. et al. Amphiphilic block copolymer-stabilized gold nanoparticles for aerobic oxidation of alcohols in aqueous solution. Chem. Commun. 4442–4444 (2008).
Fristrup, P., Johansen, L. B. & Christensen, C. H. Mechanistic investigation of the gold-catalyzed aerobic oxidation of alcohols. Catal. Lett. 120, 184–190 (2008).
Fristrup, P., Johansen, L. B. & Christensen, C. H. Mechanistic investigation of the gold-catalyzed aerobic oxidation of aldehydes: added insight from Hammett studies and isotopic labelling experiments. Chem. Commun. 2750–2752 (2008).
Abad, A., Almela, C., Corma, A. & Garcia, H. Efficient chemoselective alcohol oxidation using oxygen as oxidant. Superior performance of gold over palladium catalysts. Tetrahedron 62, 6666–6672 (2006).
Nielsen, I. S., Taarning, E., Egeblad, K., Madsen, R. & Christensen, C. H. Direct aerobic oxidation of primary alcohols to methyl esters catalyzed by a heterogeneous gold catalyst. Catal. Lett. 116, 35–40 (2007).
Xu, B., Liu, X., Haubrich, J., Madix, R. J. & Friend, C. M. Selectivity control in gold-mediated esterification of methanol. Angew. Chem. Int. Ed. 48, 4206–4209 (2009).
Liu, X., Xu, B., Haubrich, J., Madix, R. J. & Friend, C. M. Surface-mediated self-coupling of ethanol on gold. J. Am. Chem. Soc. 131, 5757–5759 (2009).
Marsden, C. et al. Aerobic oxidation of aldehydes under ambient conditions using supported gold nanoparticle catalysts. Green Chem. 10, 168–170 (2008).
Mancuso, A. J., Huang, S. L. & Swern, D. Oxidation of long-chain and related alcohols to carbonyls by dimethyl-sulfoxide activated by oxalyl chloride. J. Org. Chem. 43, 2480–2482 (1978).
Andreasen, A., Lynggaard, H., Stegelmann, C. & Stoltze, P. A microkinetic model of the methanol oxidation over silver. Surf. Sci. 544, 5–23 (2003).
Lambert, R. M., Williams, F. J., Cropley, R. L. & Palermo, A. Heterogeneous alkene epoxidation: past, present and future. J. Mol. Catal. A 228, 27–33 (2005).
Liu, X. Y., Madix, R. J. & Friend, C. M. Unraveling molecular transformations on surfaces: a critical comparison of oxidation reactions on coinage metals. Chem. Soc. Rev. 37, 2243–2261 (2008).
Wachs, I. E. & Madix, R. J. Selective oxidation of CH3OH to H2CO on a copper(110) catalyst. J. Catal. 53, 208–227 (1978).
Wachs, I. E. & Madix, R. J. Oxidation of methanol on a silver (110) catalyst. Surf. Sci. 76, 531–558 (1978).
Madix, R. J. Molecular-transformations on single-crystal metal-surfaces. Science 233, 1159–1166 (1986).
Biella, S., Castiglioni, G. L., Fumagalli, C., Prati, L. & Rossi, M. Application of gold catalysts to selective liquid phase oxidation. Catal. Today. 72, 242–247 (2002).
Biella, S., Prati, L. & Rossi, M. Gold catalyzed oxidation of aldehydes in liquid phase. J. Mol. Catal. A 197, 378–379 (2003).
Wang, X. G. et al. Selective oxidation of alcohols to aldehydes and ketones over TiO2-supported gold nanoparticles in supercritical carbon dioxide with molecular oxygen. Appl. Catal. A 349, 86–90 (2008).
Min, B. K., Alemozafar, A. R., Pinnaduwage, D., Deng, X. & Friend, C. M. Efficient CO oxidation at low temperature on Au(111). J. Phys. Chem. B 110, 19833–9838 (2006).
Madix, R. J., Friend, C. M. & Liu, X. Y. Anticipating catalytic oxidation reactions on gold at high pressure (including liquid phase) from ultrahigh vacuum studies. J. Catal. 258, 410–413 (2008).
Saliba, N., Parker, D. H. & Koel, B. E. Adsorption of oxygen on Au(111) by exposure to ozone. Surf. Sci. 410, 270–282 (1998).
Benziger, J. B., Ko, E. I. & Madix, R. J. Reactions of formaldehyde on W(100) and W(100)-(5x1)C. J. Catal. 64, 132–142 (1980).
Stein, S. E. in NIST Chemistry WebBook (eds Linstrom, P. J. and Mallard, W. G.), NIST standard reference database number 69 (National Institute of Standards and Technology, 2009); available at <http://webbook.nist.gov>.
Acknowledgements
We gratefully acknowledge the support of this work by the US Department of Energy, Basic Energy Sciences, under Grant No. FG02-84-ER13289. J.H. (Feodour-Lynen fellowship) acknowledges support through the A. v. Humboldt foundation. Correspondence and requests for materials should be addressed to C.F.
Author information
Authors and Affiliations
Contributions
B.X., X.L., J.H. and C.F. conceived and designed experiments, analysed and discussed results, and commented on the manuscript. B.X. performed the experiments and analysed data. B.X. and C.F. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 360 kb)
Rights and permissions
About this article
Cite this article
Xu, B., Liu, X., Haubrich, J. et al. Vapour-phase gold-surface-mediated coupling of aldehydes with methanol. Nature Chem 2, 61–65 (2010). https://doi.org/10.1038/nchem.467
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.467
This article is cited by
-
Octahedral gold-silver nanoframes with rich crystalline defects for efficient methanol oxidation manifesting a CO-promoting effect
Nature Communications (2019)
-
Structural Differentiation of the Reactivity of Alcohols with Active Oxygen on Au(110)
Topics in Catalysis (2018)
-
Direct Formation of Acetate from the Partial Oxidation of Ethylene on a Au/TiO2 Catalyst
Topics in Catalysis (2013)
-
The promoting effect of adsorbed carbon monoxide on the oxidation of alcohols on a gold catalyst
Nature Chemistry (2012)