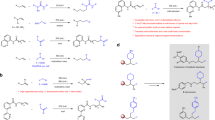
Abstract
Polyoxygenated hydrocarbons that bear one or more hydroxyl groups comprise a large set of natural and synthetic compounds, often with potent biological activity. In synthetic chemistry, alcohols are important precursors to carbonyl groups, which then can be converted into a wide range of oxygen- or nitrogen-based functionality. Therefore, the selective conversion of a single hydroxyl group in natural products into a ketone would enable the selective introduction of unnatural functionality. However, the methods known to convert a simple alcohol, or even an alcohol in a molecule that contains multiple protected functional groups, are not suitable for selective reactions of complex polyol structures. We present a new ruthenium catalyst with a unique efficacy for the selective oxidation of a single hydroxyl group among many in unprotected polyol natural products. This oxidation enables the introduction of nitrogen-based functional groups into such structures that lack nitrogen atoms and enables a selective alcohol epimerization by stepwise or reversible oxidation and reduction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lewis, C. A. & Miller, S. J. Site-selective derivatization and remodeling of erythromycin A by using simple peptide-based chiral catalysts. Angew. Chem. Int. Ed. 45, 5616–5619 (2006).
Sun, X., Lee, H., Lee, S. & Tan, K. L. Catalyst recognition of cis-1,2-diols enables site-selective functionalization of complex molecules. Nat. Chem. 5, 790–795 (2013).
Peddibhotla, S., Dang, Y., Liu, J. O. & Romo, D. Simultaneous arming and structure/activity studies of natural products employing O–H insertions: an expedient and versatile strategy for natural products-based chemical genetics. J. Am. Chem. Soc. 129, 12222–12231 (2007).
Dobereiner, G. E. & Crabtree, R. H. Dehydrogenation as a substrate-activating strategy in homogeneous transition-metal catalysis. Chem. Rev. 110, 681–703 (2010).
Noyori, R. & Hashiguchi, S. Asymmetric transfer hydrogenation catalyzed by chiral ruthenium complexes. Acc. Chem. Res. 30, 97–102 (1997).
Shvo, Y., Czarkie, D. & Rahamim, Y. A new group of ruthenium complexes: structure and catalysis. J. Am. Chem. Soc. 108, 7400–7402 (1986).
Almeida, M. L. S., Beller, M., Wang, G. & Backvall, J. Ruthenium(II)-catalyzed Oppenauer-type oxidation of secondary alcohols. Chem.-Eur. J. 1533–1536 (1996).
Oldenhuis, N. J., Dong, V. M. & Guan, Z. From racemic alcohols to enantiopure amines: Ru-catalyzed diastereoselective amination. J. Am. Chem. Soc. 136, 12548–12551 (2014).
Xiang, Y., Zhang, H., Fan, C. Q. & Yue, J. M. Novel diterpenoids and diterpenoid glycosides from Siegesbeckia orientalis. J. Nat. Prod. 67, 1517–1521 (2004).
Arnone, A. et al. Microbial transformation of 10-deacetylbaccatin III (10-DAB) by Curvularia lunata and Trametes hirsuta. J. Mol. Catal. B 42, 95–98 (2006).
Luo, J. et al. Synthesis of stable genipin derivatives and studies of their neuroprotective activity in PC12 cells. ChemMedChem 7, 1661–1668 (2012).
Safaev, M. A., Zainutdinov, U. N. & Aslanov, K. A. Continuous method for obtaining lagochirzen. Chem. Nat. Compd 31, 145 (1995).
Watanabe, M., Tanaka, K., Miki, T. & Murata, K. Process for preparing amine compound. US patent 2012/0065426 A1 (2012).
Gavagnin, R., Cataldo, M., Pinna, F. & Strukul, G. Diphosphine−palladium and −platinum complexes as catalysts for the Baeyer−Villiger oxidation of ketones: effect of the diphosphine, oxidation of acyclic ketones, and mechanistic studies. Organometallics 17, 661–667 (1998).
Pàmies, O. & Bäckvall, J. E. Combination of enzymes and metal catalysts. A powerful approach in asymmetric catalysis. Chem. Rev. 103, 3247–3261 (2003).
Warner, M. C. & Backvall, J. E. Mechanistic aspects on cyclopentadienylruthenium complexes in catalytic racemization of alcohols. Acc. Chem. Res. 46, 2545–2555 (2013).
Långvik, O., Mavrynsky, D. & Leino, R. Selective ruthenium-catalyzed epimerization of chiral sec-alcohols. Catal. Today 241, 255–259 (2015).
Mallat, T. & Baiker, A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Rev. 104, 3037–3058 (2004).
Kwon, M. S. et al. Palladium nanoparticles entrapped in aluminum hydroxide: dual catalyst for alkene hydrogenation and aerobic alcohol oxidation. Org. Lett. 7, 1077–1079 (2005).
Steves, J. E. & Stahl, S. S. Stable TEMPO and ABNO catalyst solutions for user-friendly (bpy)Cu/nitroxyl-catalyzed aerobic alcohol oxidation. J. Org. Chem. 80, 11184–11188 (2015).
Kim, W. H., Park, I. S. & Park, J. Acceptor-free alcohol dehydrogenation by recyclable ruthenium catalyst. Org. Lett. 8, 2543–2545 (2006).
Yi, C. S., Zeczycki, T. N. & Guzei, I. A. Highly cooperative tetrametallic ruthenium-μ-oxo-μ-hydroxo catalyst for the alcohol oxidation reaction. Organometallics 25, 1047–1051 (2006).
Eisink, N. N. H. M., Lohse, J., Witte, M. D. & Minnaard, A. J. Regioselective oxidation of unprotected 1,4 linked glucans. Org. Biomol. Chem. 14, 4859–4864 (2016).
Scott, R. W. et al. Mupirocin F: structure elucidation, synthesis and rearrangements. Tetrahedron 67, 5098–5106 (2011).
Bhat, S. V., Bajwa, B. S., Dornauer, H. & de Souza, N. J. Reactions of forskolin, a biologically active diterpenoid from coleus forskohlii. J. Chem. Soc. Perkin Trans. 1 767–771 (1982).
Mathad, V. T., Kumar, S. & Raj, K. Oxidation studies on andrographolide. Nat. Prod. Res. 20, 1053–1058 (2006).
Hayashi, M., Yamada, K. & Nakayama, S. Dehydrogenation of D-glycals by palladium supported on activated charcoal under ethylene atmosphere: synthesis of 1,5-anhydrohex-1-en-3-uloses. Synthesis 1869–1871 (1999).
Fieser, L. F. & Rajagopalan, S. Selective oxidation with N-bromosuccinimide. I. cholic acid. J. Am. Chem. Soc. 71, 3935–3938 (1949).
Welankiwar, S. S. & Murphy, W. S. Stereoselective oxidation of fusidic acid derivatives. J. Chem. Soc. Perkin Trans. 1 710–712 (1976).
Chung, K. et al. Chemoselective Pd-catalyzed oxidation of polyols: synthetic scope and mechanistic studies. J. Am. Chem. Soc. 135, 7593–7602 (2013).
Yi, C. S., He, Z. & Guzei, I. A. Transfer hydrogenation of carbonyl compounds catalyzed by a ruthenium-acetamido complex: evidence for a stepwise hydrogen transfer mechanism. Organometallics 20, 3641–3643 (2001).
Acknowledgements
We thank the NIH (GM-55382) and the Berkeley Center for Green Chemistry Systems Approach to Green Energy Integrated Graduate Education and Research Traineeship for financial support. We acknowledge A. DiPasquale for assistance with X-ray crystallographic characterizations and the NIH (SIG S10-RR027172) for facility funding.
Author information
Authors and Affiliations
Contributions
C.K.H. and J.F.H. conceived and designed the experiments. C.K.H. performed the experiments. C.K.H. and J.F.H. analysed the data. C.K.H. and J.F.H. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2520 kb)
Supplementary information
Crystallographic data for compound 1l (CIF 1908 kb)
Supplementary information
Crystallographic data for compound 2c (CIF 382 kb)
Supplementary information
Crystallographic data for compound 2d (CIF 406 kb)
Supplementary information
Crystallographic data for compound 2e (CIF 540 kb)
Supplementary information
Crystallographic data for compound Ru-2-DABIII-Alkoxid (CIF 2225 kb)
Supplementary information
Crystallographic data for compound Ru-2 (CIF 2846 kb)
Supplementary information
Crystallographic data for compound Ru-3-Cl (CIF 725 kb)
Rights and permissions
About this article
Cite this article
Hill, C., Hartwig, J. Site-selective oxidation, amination and epimerization reactions of complex polyols enabled by transfer hydrogenation. Nature Chem 9, 1213–1221 (2017). https://doi.org/10.1038/nchem.2835
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2835
This article is cited by
-
Biomimetic caged platinum catalyst for hydrosilylation reaction with high site selectivity
Nature Communications (2021)
-
Dual-catalytic transition metal systems for functionalization of unreactive sites of molecules
Nature Catalysis (2019)
-
A general strategy for diversifying complex natural products to polycyclic scaffolds with medium-sized rings
Nature Communications (2019)