Abstract
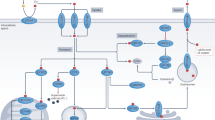
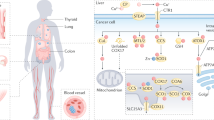
Copper is a transition metal that plays critical roles in many life processes. Controlling the cellular concentration and trafficking of copper offers a route to disrupt these processes. Here we report small molecules that inhibit the human copper-trafficking proteins Atox1 and CCS, and so provide a selective approach to disrupt cellular copper transport. The knockdown of Atox1 and CCS or their inhibition leads to a significantly reduced proliferation of cancer cells, but not of normal cells, as well as to attenuated tumour growth in mouse models. We show that blocking copper trafficking induces cellular oxidative stress and reduces levels of cellular ATP. The reduced level of ATP results in activation of the AMP-activated protein kinase that leads to reduced lipogenesis. Both effects contribute to the inhibition of cancer cell proliferation. Our results establish copper chaperones as new targets for future developments in anticancer therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Labbe, S. & Thiele, D. J. Pipes and wiring: the regulation of copper uptake and distribution in yeast. Trends Microbiol. 7, 500–505 (1999).
Kim, B. E., Nevitt, T. & Thiele, D. J. Mechanisms for copper acquisition, distribution and regulation. Nature Chem. Biol. 4, 176–185 (2008).
O'Halloran, T. V. & Culotta, V. C. Metallochaperones, an intracellular shuttle service for metal ions. J. Biol. Chem. 275, 25057–25060 (2000).
Rosenzweig, A. C. Copper delivery by metallochaperone proteins. Acc. Chem. Res. 34, 119–128 (2001).
Lutsenko, S., Gupta, A., Burkhead, J. L. & Zuzel, V. Cellular multitasking: the dual role of human Cu-ATPases in cofactor delivery and intracellular copper balance. Arch. Biochem. Biophys. 476, 22–32 (2008).
La Fontaine, S. & Mercer, J. F. Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch. Biochem. Biophys. 463, 149–167 (2007).
Rae, T. D., Schmidt, P. J., Pufahl, R. A., Culotta, V. C. & O'Halloran, T. V. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284, 805–808 (1999).
Banci, L. et al. Human superoxide dismutase 1 (hSOD1) maturation through interaction with human copper chaperone for SOD1 (hCCS). Proc. Natl Acad. Sci. USA 109, 13555–13560 (2012).
Caruano-Yzermans, A. L. et al. Mechanisms of the copper-dependent turnover of the copper chaperone for superoxide dismutase. J. Biol. Chem. 281, 13581–13587 (2006).
Rae, T. D. et al. Mechanism of Cu,Zn-superoxide dismutase activation by the human metallochaperone hCCS. J. Biol. Chem. 276, 5166–5176 (2001).
Lamb, A. L. et al. Crystal structure of the copper chaperone for superoxide dismutase. Nature Struct. Biol. 6, 724–729 (1999).
Wernimont, A. K., Huffman, D. L., Lamb, A. L., O'Halloran, T. V. & Rosenzweig, A. C. Structural basis for copper transfer by the metallochaperone for the Menkes/Wilson disease proteins. Nature Struct. Biol. 7, 766–771 (2000).
Anastassopoulou, I. et al. Solution structure of the apo and copper(I)-loaded human metallochaperone HAH1. Biochemistry 43, 13046–13053 (2004).
Lamb, A. L., Torres, A. S., O'Halloran, T. V. & Rosenzweig, A. C. Heterodimeric structure of superoxide dismutase in complex with its metallochaperone. Nature Struct. Biol. 8, 751–755 (2001).
Kawamata, H. & Manfredi, G. Different regulation of wild-type and mutant Cu,Zn superoxide dismutase localization in mammalian mitochondria. Hum. Mol. Genet. 17, 3303–3317 (2008).
Valentine, J. S. & Hart, P. J. Misfolded CuZnSOD and amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA 100, 3617–3622 (2003).
Mandinov, L. et al. Copper chelation represses the vascular response to injury. Proc. Natl Acad. Sci. USA 100, 6700–6705 (2003).
Eatock, M. M., Schatzlein, A. & Kaye, S. B. Tumour vasculature as a target for anticancer therapy. Cancer Treat. Rev. 26, 191–204 (2000).
Lowndes, S. A. & Harris, A. L. The role of copper in tumour angiogenesis. J. Mammary Gland Biol. Neoplasia 10, 299–310 (2005).
Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Med. 1, 27–31 (1995).
Gupte, A. & Mumper, R. J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 35, 32–46 (2009).
Cai, H. & Peng, F. Knockdown of copper chaperone antioxidant-1 by RNA interference inhibits copper-stimulated proliferation of non-small cell lung carcinoma cells. Oncol. Rep. 30, 269–275 (2013).
Boal, A. K. & Rosenzweig, A. C. Structural biology of copper trafficking. Chem. Rev. 109, 4760–4779 (2009).
Lutsenko, S. Human copper homeostasis: a network of interconnected pathways. Curr. Opin. Chem. Biol. 14, 211–217 (2010).
Wing, T. J., Makino, S., Skillman, A. G. & Kuntz, I. D. DOCK 4.0: search strategies for automated molecular docking of flexible molecule databases. J. Comput. Aided Mol. Des. 15, 411–428 (2001).
Vinkenborg, J. L. et al. Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nature Methods 6, 737–740 (2009).
Hamza, I. et al. The metallochaperone Atox1 plays a critical role in perinatal copper homeostasis. Proc. Natl Acad. Sci. USA 98, 6848–6852 (2001).
Itoh, S. et al. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J. Biol. Chem. 283, 9157–9167 (2008).
Martinez Molina, D. et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 341, 84–87 (2013).
Gad, H. et al. MTH1 inhibition eradicates cancer by preventing sanitation of the dNTP pool. Nature 508, 215–221 (2014).
Huber, K. V. et al. Stereospecific targeting of MTH1 by (S)-crizotinib as an anticancer strategy. Nature 508, 222–227 (2014).
Sena, L. A. & Chandel, N. S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 48, 158–167 (2012).
Kroemer, G. & Pouyssegur, J. Tumor cell metabolism: cancer's Achilles’ heel. Cancer Cell 13, 472–482 (2008).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Cairns, R. A., Harris, I. S. & Mak, T. W. Regulation of cancer cell metabolism. Nature Rev. Cancer 11, 85–95 (2011).
Leary, S. C. et al. ‘Pulling the plug’ on cellular copper: the role of mitochondria in copper export. Biochim. Biophys. Acta 1793, 146–153 (2009).
Sauer, S. W. et al. Severe dysfunction of respiratory chain and cholesterol metabolism in Atp7b(–/–) mice as a model for Wilson disease. Biochim. Biophys. Acta 1812, 1607–1615 (2011).
Mihaylova, M. M. & Shaw, R. J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature Cell Biol. 13, 1016–1023 (2011).
Hardie, D. G., Ross, F. A. & Hawley, S. A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature Rev. Mol. Cell Biol. 13, 251–262 (2012).
Carracedo, A., Cantley, L. C. & Pandolfi, P. P. Cancer metabolism: fatty acid oxidation in the limelight. Nature Rev. Cancer 13, 227–232 (2013).
Schulze, A. & Harris, A. L. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature 491, 364–373 (2012).
Trachootham, D., Alexandre, J. & Huang, P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nature Rev. Drug Discov. 8, 579–591 (2009).
Raj, L. et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 475, 231–234 (2011).
Gorrini, C., Harris, I. S. & Mak, T. W. Modulation of oxidative stress as an anticancer strategy. Nature Rev. Drug Discov. 12, 931–947, (2013).
Brady, D. C. et al. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 509, 492–496 (2014).
Ishida, S. et al. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc. Natl Acad. Sci. USA 110, 19507–19512 (2013).
Santini, C. et al. Advances in copper complexes as anticancer agents. Chem. Rev. 114, 815–862 (2013).
Maryon, E. B. et al. Cellular glutathione plays a key role in copper uptake mediated by human copper transporter 1. Am. J. Physiol. Cell Physiol. 304, C768–C779 (2013).
Morris, G. M. et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791 (2009).
Gong, J. et al. ChemMapper: a versatile web server for exploring pharmacology and chemical structure association based on molecular 3D similarity method. Bioinformatics 29, 1827–1829 (2013).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (21210003 to H.J. and C.H., and 81230076, 91313000 to H.J.), the Hi-Tech Research and Development Program of China (2012AA020302 and 2012AA01A305 to C.L.), Chinese Academy of Sciences (XDA01040305 to C.L.), National Science Foundation (CHE-1213598 to C.H.) and National Institutes of Health (CA140515 to J.C.). C.H. is supported by the Howard Hughes Medical Institute as an investigator. We thank S. F. Reichard for help with editing the manuscript.
Author information
Authors and Affiliations
Contributions
C.H. conceived the project with H.J. and J.C., and J.W., C.Luo, C.S. and Q.Y. designed and performed most of the experiments. J.L. and S.O. performed the virtual screening and bioinformatics analysis. S.E., J.F. and H.K. assisted with the cell and mice experiments. Y.Z. and H.L. assisted in the synthesis of the compounds. J.L.V. and M.M. assisted in setting up the FRET-based compound screening, Y.W. and N.Z. conducted the NMR experimental and data analysis of DC_AC2 with Atox1. C.Luan, H.D. and S.C. performed the SPR experiments for DC_AC50 with Atox1 and CCS. C.H. and J.W. wrote the manuscript with input from H.J. and J.C.
Corresponding authors
Ethics declarations
Competing interests
A patent application on DC_AC50 has been filed by the University of Chicago and the Shanghai Institute of Materia Medica.
Supplementary information
Supplementary information
Supplementary information (PDF 3391 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Luo, C., Shan, C. et al. Inhibition of human copper trafficking by a small molecule significantly attenuates cancer cell proliferation. Nature Chem 7, 968–979 (2015). https://doi.org/10.1038/nchem.2381
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2381
This article is cited by
-
Deep learning enables the discovery of a novel cuproptosis-inducing molecule for the inhibition of hepatocellular carcinoma
Acta Pharmacologica Sinica (2024)
-
Iron and copper: critical executioners of ferroptosis, cuproptosis and other forms of cell death
Cell Communication and Signaling (2023)
-
Elesclomol, a copper-transporting therapeutic agent targeting mitochondria: from discovery to its novel applications
Journal of Translational Medicine (2023)
-
Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies
Cell Death & Disease (2023)
-
Programmed cell death in hepatic fibrosis: current and perspectives
Cell Death Discovery (2023)