Abstract
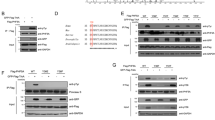
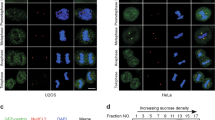
Deregulated centrosome duplication can result in genetic instability and contribute to tumorigenesis1,2. Here, we show that centrosome duplication is regulated by the activity of an E3-ubiquitin ligase that employs the F-box protein FBXW5 (ref. 3) as its targeting subunit. Depletion of endogenous FBXW5 or overexpression of an F-box-deleted mutant version results in centrosome overduplication and formation of multipolar spindles. We identify the centriolar protein HsSAS-6 (refs 4, 5) as a critical substrate of the SCF–FBXW5 complex. FBXW5 binds HsSAS-6 and promotes its ubiquitylation in vivo. The activity of SCF–FBXW5 is in turn negatively regulated by Polo-like kinase 4 (PLK4), which phosphorylates FBXW5 at Ser 151 to suppress its ability to ubiquitylate HsSAS-6. FBXW5 is a cell-cycle-regulated protein with expression levels peaking at the G1/S transition. We show that FBXW5 levels are controlled by the anaphase-promoting (APC/C) complex, which targets FBXW5 for degradation during mitosis and G1, thereby helping to reset the centrosome duplication machinery. In summary, we show that a cell-cycle-regulated SCF complex is regulated by the kinase PLK4, and that this in turn restricts centrosome re-duplication through degradation of the centriolar protein HsSAS-6.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
06 July 2011
In the version of this article initially published online, all proteins should have been upper case. This has been corrected in both the HTML and PDF versions of the article.
References
Nigg, E. A. & Raff, J. W. Centrioles, centrosomes, and cilia in health and disease. Cell 139, 663–678 (2009).
Ganem, N. J., Godinho, S. A. & Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 460, 278–282 (2009).
Hu, J. et al. WD40 protein FBW5 promotes ubiquitination of tumor suppressor TSC2 by DDB1-CUL4-ROC1 ligase. Genes Dev. 22, 866–871 (2008).
Leidel, S., Delattre, M., Cerutti, L., Baumer, K. & Gönczy, P. SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nat. Cell Biol. 7, 115–125 (2005).
Dammermann, A. et al. Centriole assembly requires both centriolar and pericentriolar material proteins. Dev. Cell 7, 815–829 (2004).
Tsou, M. F. & Stearns, T. Mechanism limiting centrosome duplication to once per cell cycle. Nature 442, 947–951 (2006).
Hinchcliffe, E. H. & Sluder, G. ‘It takes two to tango’: understanding how centrosome duplication is regulated throughout the cell cycle. Genes Dev. 15, 1167–1181 (2001).
Kleylein-Sohn, J. et al. Plk4-induced centriole biogenesis in human cells. Dev. Cell 13, 190–202 (2007).
Holland, A. J., Lan, W., Niessen, S., Hoover, H. & Cleveland, D. W. Polo-like kinase 4 kinase activity limits centrosome overduplication by autoregulating its own stability. J. Cell Biol. 188, 191–198 (2010).
Habedanck, R., Stierhof, Y. D., Wilkinson, C. J. & Nigg, E. A. The Polo kinase Plk4 functions in centriole duplication. Nat. Cell Biol. 7, 1140–1146 (2005).
Kohlmaier, G. et al. Overly long centrioles and defective cell division on excess of the SAS-4-related protein CPAP. Curr. Biol. 19, 1012–1018 (2009).
Schmidt, T. I. et al. Control of centriole length by CPAP and CP110. Curr. Biol. 19, 1005–1011 (2009).
Delattre, M. et al. Centriolar SAS-5 is required for centrosome duplication in C. elegans. Nat. Cell Biol. 6, 656–664 (2004).
Tang, C. J., Fu, R. H., Wu, K. S., Hsu, W. B. & Tang, T. K. CPAP is a cell-cycle regulated protein that controls centriole length. Nat. Cell Biol. 11, 825–831 (2009).
Skaar, J. R. & Pagano, M. Control of cell growth by the SCF and APC/C ubiquitin ligases. Curr. Opin. Cell Biol. 21, 816–824 (2009).
Petroski, M. D. & Deshaies, R. J. Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 6, 9–20 (2005).
Jin, J. et al. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 18, 2573–2580 (2004).
Meraldi, P., Honda, R. & Nigg, E. A. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53-/- cells. EMBO J. 21, 483–492 (2002).
Freed, E. et al. Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle. Genes Dev. 13, 2242–2257 (1999).
Korzeniewski, N. et al. Cullin 1 functions as a centrosomal suppressor of centriole multiplication by regulating polo-like kinase 4 protein levels. Cancer Res. 69, 6668–6675 (2009).
Yamano, H., Tsurumi, C., Gannon, J. & Hunt, T. The role of the destruction box and its neighbouring lysine residues in cyclin B for anaphase ubiquitin-dependent proteolysis in fission yeast: defining the D-box receptor. EMBO J. 17, 5670–5678 (1998).
Strnad, P. et al. Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev. Cell 13, 203–213 (2007).
Johnson, E. F., Stewart, K. D., Woods, K. W., Giranda, V. L. & Luo, Y. Pharmacological and functional comparison of the polo-like kinase family: insight into inhibitor and substrate specificity. Biochemistry 46, 9551–9563 (2007).
Sillibourne, J. E. et al. Autophosphorylation of polo-like kinase 4 and its role in centriole duplication. Mol. Biol. Cell 21, 547–561 (2010).
D’Angiolella, V. et al. SCF(Cyclin F) controls centrosome homeostasis and mitotic fidelity through CP110 degradation. Nature 466, 138–142 (2010).
Malek, N. P. et al. A mouse knock-in model exposes sequential proteolytic pathways that regulate p27Kip1 in G1 and S phase. Nature 413, 323–327 (2001).
Moudjou, M. & Bornens, M. Cell Biology: A Laboratory Handbook (Academic, 1994).
Campanero, M. R. & Flemington, E. K. Regulation of E2F through ubiquitin-proteasome-dependent degradation: stabilization by the pRB tumor suppressor protein. Proc. Natl Acad. Sci. USA 94, 2221–2226 (1997).
Acknowledgements
We thank H. S. Malek, J. Roberts and A. Besson for critical reading of the manuscript. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (Transregio 77) to N.P.M., and by a grant from the Deutsche Krebshilfe to V.G. and S.K., as well as by the Swiss Cancer League to P.G. (OCS KLS 02024-02-2007).
Author information
Authors and Affiliations
Contributions
A.P., Y.H., D.K., M.M., S.C., U.K. and A.P. carried out experiments, analysed data and designed figures; V.G., S.K., A.P., M.P.M., I.H., P.G. and N.P.M. designed experiments, analysed data, designed figures and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1903 kb)
Rights and permissions
About this article
Cite this article
Puklowski, A., Homsi, Y., Keller, D. et al. The SCF–FBXW5 E3-ubiquitin ligase is regulated by PLK4 and targets HsSAS-6 to control centrosome duplication. Nat Cell Biol 13, 1004–1009 (2011). https://doi.org/10.1038/ncb2282
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2282
This article is cited by
-
Prolonged overexpression of PLK4 leads to formation of centriole rosette clusters that are connected via canonical centrosome linker proteins
Scientific Reports (2024)
-
Genome-wide analysis of genes encoding core components of the ubiquitin system during cerebral cortex development
Molecular Brain (2022)
-
The E3 ubiquitin ligase, FBXW5, promotes the migration and invasion of gastric cancer through the dysregulation of the Hippo pathway
Cell Death Discovery (2022)
-
NudC-like protein 2 restrains centriole amplification by stabilizing HERC2
Cell Death & Disease (2019)
-
Caspase-mediated cleavage of the centrosomal proteins during apoptosis
Cell Death & Disease (2018)