Abstract
Misfolding and aggregation of proteins containing expanded polyglutamine repeats underlie Huntington's disease and other neurodegenerative disorders1. Here, we show that the hetero-oligomeric chaperonin TRiC (also known as CCT) physically interacts with polyglutamine-expanded variants of huntingtin (Htt) and effectively inhibits their aggregation. Depletion of TRiC enhances polyglutamine aggregation in yeast and mammalian cells. Conversely, overexpression of a single TRiC subunit, CCT1, is sufficient to remodel Htt-aggregate morphology in vivo and in vitro, and reduces Htt-induced toxicity in neuronal cells. Because TRiC acts during de novo protein biogenesis2, this chaperonin may have an early role preventing Htt access to pathogenic conformations. Based on the specificity of the Htt–CCT1 interaction, the CCT1 substrate-binding domain may provide a versatile scaffold for therapeutic inhibitors of neurodegenerative disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zoghbi, H. Y. & Orr, H. T. Glutamine repeats and neurodegeneration. Annu. Rev. Neurosci. 23, 217–247 (2000).
Frydman, J., Nimmesgern, E., Ohtsuka, K. & Hartl, F. U. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature 370, 111–117 (1994).
Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nature Rev. Neurosci. 4, 49–60 (2003).
Muchowski, P. J. & Wacker, J. L. Modulation of neurodegeneration by molecular chaperones. Nature Rev. Neurosci. 6, 11–22 (2005).
Scherzinger, E. et al. Self-assembly of polyglutamine-containing huntingtin fragments into amyloid-like fibrils: implications for Huntington's disease pathology. Proc. Natl Acad. Sci. USA 96, 4604–4609 (1999).
Frydman, J. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem. 70, 603–647 (2001).
Hartl, F. U. & Hayer-Hartl, M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295, 1852–1858 (2002).
Nollen, E. A. et al. Genome-wide RNA interference screen identifies previously undescribed regulators of polyglutamine aggregation. Proc. Natl Acad. Sci. USA 101, 6403–6408 (2004).
Spiess, C., Meyer, A. S., Reissmann, S. & Frydman, J. Mechanism of the eukaryotic chaperonin: protein folding in the chamber of secrets. Trends Cell Biol. 14, 598–604 (2004).
Melville, M. W., McClellan, A. J., Meyer, A. S., Darveau, A. & Frydman, J. The Hsp70 and TRiC/CCT chaperone systems cooperate in vivo to assemble the von Hippel-Lindau tumor suppressor complex. Mol. Cell Biol. 23, 3141–3151 (2003).
Krobitsch, S. & Lindquist, S. Aggregation of huntingtin in yeast varies with the length of the polyglutamine expansion and the expression of chaperone proteins. Proc. Natl Acad. Sci. USA 97, 1589–1594 (2000).
Vinh, D. B. & Drubin, D. G. A yeast TCP-1-like protein is required for actin function in vivo. Proc. Natl Acad. Sci. USA 91, 9116–9120 (1994).
Camasses, A., Bogdanova, A., Shevchenko, A. & Zachariae, W. The CCT chaperonin promotes activation of the anaphase-promoting complex through the generation of functional Cdc20. Mol. Cell 12, 87–100 (2003).
Deutschbauer, A. M. et al. Mechanisms of haploinsufficiency revealed by genome-wide profiling in yeast. Genetics 169, 1915–1925 (2005).
Muchowski, P. J. et al. Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc. Natl Acad. Sci. USA 97, 7841–7846 (2000).
Muchowski, P. J., Ning, K., D' Souza-Schorey, C. & Fields, S. Requirement of an intact microtubule cytoskeleton for aggregation and inclusion body formation by a mutant huntingtin fragment. Proc. Natl Acad. Sci. USA 99, 727–732 (2002).
Kayed, R. et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489 (2003).
Jana, N. R., Tanaka, M., Wang, G. & Nukina, N. Polyglutamine length-dependent interaction of Hsp40 and Hsp70 family chaperones with truncated N-terminal huntingtin: their role in suppression of aggregation and cellular toxicity. Hum. Mol. Genet. 9, 2009–2018 (2000).
Albanese, V., Yam, A. Y., Baughman, J., Parnot, C. & Frydman, J. Systems analyses reveal two chaperone networks with distinct functions in eukaryotic cells. Cell 124, 75–88 (2006).
Arrasate, M., Mitra, S., Schweitzer, E. S., Segal, M. R. & Finkbeiner, S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431, 805–810 (2004).
Poirier, M. A. et al. Huntingtin spheroids and protofibrils as precursors in polyglutamine fibrilization. J. Biol. Chem. 277, 41032–41037 (2002).
Wacker, J. L., Zareie, M. H., Fong, H., Sarikaya, M. & Muchowski, P. J. Hsp70 and Hsp40 attenuate formation of spherical and annular polyglutamine oligomers by partitioning monomer. Nature Struct. Mol. Biol. 11, 1215–1222 (2004).
Marx, J. Neurodegeneration. Huntington's research points to possible new therapies. Science 310, 43–45 (2005).
Adams, A., Gottschling, D. E., Daiser, C. A. & Stearns, T. Methods in Yeast Genetics (Cold Spring Harbor Laboratory Press, New York, 1997).
Bence, N. F., Sampat, R. M. & Kopito, R. R. Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292, 1552–1555 (2001).
Bennett, E. J., Bence, N. F., Jayakumar, R. & Kopito, R. R. Global impairment of the ubiquitin–proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol. Cell 17, 351–365 (2005).
Kabir, M. A. et al. Physiological effects of unassembled chaperonin Cct subunits in the yeast Saccharomyces cerevisiae. Yeast 22, 219–239 (2005).
McClellan, A. J., Scott, M. D. & Frydman, J. Folding and quality control of the VHL tumor suppressor proceed through distinct chaperone pathways. Cell 121, 739–748 (2005).
Parran, D. K., Barker, A. & Ehrich, M. Effects of thimerosal on NGF signal transduction and cell death in neuroblastoma cells. Toxicol. Sci. 86, 132–140 (2005).
Ferreyra, R. G. & Frydman, J. Purification of the cytosolic chaperonin TRiC from bovine testis. Methods Mol. Biol. 140, 153–160 (2000).
Acknowledgements
We thank W. Zachariae, R. Kopito, R. Davis, N. Nukina and F. Sherman for kindly providing reagents and cells, P. Ren, V. Albanese, B. Riley, A.J. McClellan and other members of the Frydman and Kopito labs for advice and stimulating discussions and R. Andino for useful discussions and comments on the manuscript. This work was supported by National Institutes of Health (NIH) grants GM56433 and GM74074.
Author information
Authors and Affiliations
Contributions
S.T. and J.F. planned the project. S.T., R.G. and C.S. prepared reagents and performed experiments. S.T., R.G., C.S. and J.F. designed experiments, interpreted data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3, S4, S5 and Supplementary table S1. (PDF 312 kb)
Rights and permissions
About this article
Cite this article
Tam, S., Geller, R., Spiess, C. et al. The chaperonin TRiC controls polyglutamine aggregation and toxicity through subunit-specific interactions. Nat Cell Biol 8, 1155–1162 (2006). https://doi.org/10.1038/ncb1477
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1477
This article is cited by
-
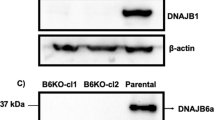
Identification of a HTT-specific binding motif in DNAJB1 essential for suppression and disaggregation of HTT
Nature Communications (2022)
-
TCP1 increases drug resistance in acute myeloid leukemia by suppressing autophagy via activating AKT/mTOR signaling
Cell Death & Disease (2021)
-
Investigating Chaperonin-Containing TCP-1 subunit 2 as an essential component of the chaperonin complex for tumorigenesis
Scientific Reports (2020)
-
Quantitative analysis of global protein stability rates in tissues
Scientific Reports (2020)
-
The role of the molecular chaperone CCT in protein folding and mediation of cytoskeleton-associated processes: implications for cancer cell biology
Cell Stress and Chaperones (2019)