Abstract
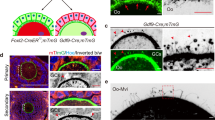
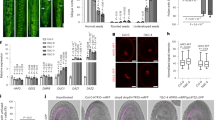
A fundamental question in animal development is how motile cells find their correct target destinations. During mating in the nematode Caenorhabditis elegans, males inject sperm through the hermaphrodite vulva into the uterus. Amoeboid sperm crawl around fertilized eggs to the spermatheca – a convoluted tube where fertilization occurs1,2. Here, we show that polyunsaturated fatty acids (PUFAs), the precursors of eicosanoid signalling molecules, function in oocytes to control directional sperm motility within the uterus. PUFAs are transported from the intestine, the site of fat metabolism, to the oocytes yolk, which is a lipoprotein complex. Loss of the RME-2 low-density lipoprotein (LDL) receptor, which mediates yolk endocytosis3 and fatty acid transport into oocytes, causes severe defects in sperm targeting. We used an RNAi screen to identify lipid regulators required for directional sperm motility. Our results support the hypothesis that PUFAs function in oocytes as precursors of signals that control sperm recruitment to the spermatheca. A common property of PUFAs in mammals and C. elegans is that these fats control local recruitment of motile cells to their target tissues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Singson, A. Every sperm is sacred: fertilization in Caenorhabditis elegans. Dev. Biol. 230, 101–109 (2001).
Ward, S. & Carrel, J. S. Fertilization and sperm competition in the nematode Caenorhabditis elegans. Dev. Biol. 73, 304–321 (1979).
Grant, B. & Hirsh, D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell 10, 4311–4326 (1999).
Kosinski, M., McDonald, K., Schwartz, J., Yamamoto, I. & Greenstein, D. C. elegans sperm bud vesicles to deliver a meiotic maturation signal to distant oocytes. Development 132, 3357–3369 (2005).
Hill, K. L. & L'Hernault, S. W. Analyses of reproductive interactions that occur after heterospecific matings within the genus Caenorhabditis. Dev. Biol. 232, 105–114 (2001).
Vacquier, V. D. Evolution of gamete recognition proteins. Science 281, 1995–1998 (1998).
Beanan, M. J. & Strome, S. Characterization of a germ-line proliferation mutation in C. elegans. Development 116, 755–766 (1992).
Barton, M. K., Schedl, T. B. & Kimble, J. Gain-of-function mutations of fem-3, a sex-determination gene in Caenorhabditis elegans. Genetics 115, 107–119 (1987).
Graham, P. L., Schedl, T. & Kimble, J. More mog genes that influence the switch from spermatogenesis to oogenesis in the hermaphrodite germ line of Caenorhabditis elegans. Dev. Genet. 14, 471–484 (1993).
Francis, R., Barton, M. K., Kimble, J. & Schedl, T. gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans. Genetics 139, 579–606 (1995).
Kurzchalia, T. V. & Ward, S. Why do worms need cholesterol? Nature Cell Biol. 5, 684–688 (2003).
Watts, J. L. & Browse, J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 99, 5854–5859 (2002).
Watts, J. L., Phillips, E., Griffing, K. R. & Browse, J. Deficiencies in C20 polyunsaturated fatty acids cause behavioral and developmental defects in Caenorhabditis elegans fat-3 mutants. Genetics 163, 581–589 (2003).
McKay, R. M., McKay, J. P., Avery, L. & Graff, J. M. C elegans: a model for exploring the genetics of fat storage. Dev. Cell 4, 131–142 (2003).
Funk, C. D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294, 1871–1875 (2001).
Kahn-Kirby, A. H. et al. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell 119, 889–900 (2004).
Reinke, V., Gil, I. S., Ward, S. & Kazmer, K. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development 131, 311–323 (2004).
Kohara, Y. Systematic analysis of gene expression of the C. elegans genome. Tanpakushitsu Kakusan Koso 46, 2425–2431 (2001).
Sijen, T. et al. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell 107, 465–476 (2001).
Sharrock, W. J., Sutherlin, M. E., Leske, K., Cheng, T. K. & Kim, T. Y. Two distinct yolk lipoprotein complexes from Caenorhabditis elegans. J. Biol. Chem. 265, 14422–14431 (1990).
Hall, D. H. et al. Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev. Biol. 212, 101–123 (1999).
Janssen-Timmen, U., Tomic, I., Specht, E., Beilecke, U. & Habenicht, A. J. The arachidonic acid cascade, eicosanoids, and signal transduction. Ann. NY Acad. Sci. 733, 325–334 (1994).
Luster, A. D. & Tager, A. M. T-cell trafficking in asthma: lipid mediators grease the way. Nature Rev. Immunol. 4, 711–724 (2004).
Tager, A. M. et al. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nature Immunol. 4, 982–990 (2003).
Goodarzi, K., Goodarzi, M., Tager, A. M., Luster, A. D. & von Andrian, U. H. Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nature Immunol. 4, 965–973 (2003).
Eisenbach, M. & Giojalas, L. C. Sperm guidance in mammals – an unpaved road to the egg. Nature Rev. Mol. Cell Biol. 7, 276–285 (2006).
Timmons, L. & Fire, A. Specific interference by ingested dsRNA. Nature 395, 854 (1998).
Merris, M. et al. Sterol effects and sites of sterol accumulation in Caenorhabditis elegans: developmental requirement for 4α-methyl sterols. J. Lipid Res. 44, 172–181 (2003).
Miller, M. A. et al. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science 291, 2144–2147 (2001).
Acknowledgements
We thank D. Greenstein, G. Marques and R. Steele for comments on the manuscript. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH). Financial support came from the University of Alabama at Birmingham (UAB), Department of Cell Biology, including Howard Hughes Medical Institute (HMMI) start-up funds delegated by UAB, UAB Comprehensive Cancer Center Junior Faculty Development and American Cancer Society Institutional Research (ACSIRG) grants to M.A.M., and an NIH grant (RO1DK074114) to J.L.W.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, Supplementary Tables S1, S2 and Supplementary Data (PDF 2361 kb)
Rights and permissions
About this article
Cite this article
Kubagawa, H., Watts, J., Corrigan, C. et al. Oocyte signals derived from polyunsaturated fatty acids control sperm recruitment in vivo. Nat Cell Biol 8, 1143–1148 (2006). https://doi.org/10.1038/ncb1476
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1476
This article is cited by
-
Dietary n-3 but not n-6 fatty acids modulate anthropometry and fertility indices in high-fat diet fed rats: a two-generation study
Journal of Food Science and Technology (2021)
-
Polyunsaturated fatty acids and p38-MAPK link metabolic reprogramming to cytoprotective gene expression during dietary restriction
Nature Communications (2020)
-
Spectroscopic coherent Raman imaging of Caenorhabditis elegans reveals lipid particle diversity
Nature Chemical Biology (2020)
-
Signature profile of cyclooxygenase-independent F2 series prostaglandins in C. elegans and their role in sperm motility
Scientific Reports (2019)
-
Specific polyunsaturated fatty acids modulate lipid delivery and oocyte development in C. elegans revealed by molecular-selective label-free imaging
Scientific Reports (2016)