Abstract
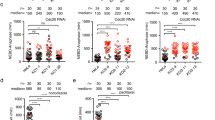
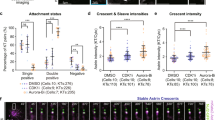
Loss or gain of whole chromosomes, the form of chromosomal instability (CIN) most commonly associated with human cancers, is expected to arise from the failure to accurately segregate chromosomes in mitosis1. The mitotic checkpoint is one pathway that prevents segregation errors by blocking the onset of anaphase until all chromosomes make proper attachments to the spindle. Another process that prevents errors is stabilization and destabilization of connections between chromosomes and spindle microtubules. An outstanding question is how these two pathways are coordinated to ensure accurate chromosome segregation. Here we show that in human cells depleted of BubR1 — a critical component of the mitotic checkpoint that can directly regulate the onset of anaphase2,3,4 — chromosomes do not form stable attachments to spindle microtubules. Attachments in these cells are restored by inhibition of Aurora kinase, which is known to stabilize kinetochore–microtubule attachments5,6,7. Loss of BubR1 function thus perturbs regulation of attachments rather than the ability of kinetochores to bind to microtubules. Consistent with this finding, depletion of BubR1 increases phosphorylation of CENP-A, a kinetochore-specific Aurora kinase substrate. We propose that BubR1 links regulation of chromosome–spindle attachment to mitotic checkpoint signalling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lengauer, C., Kinzler, K. W. & Vogelstein, B. Genetic instabilities in human cancers. Nature 396, 643–649 (1998).
Sudakin, V., Chan, G. K. & Yen, T. J. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 154, 925–936 (2001).
Tang, Z., Bharadwaj, R., Li, B. & Yu, H. Mad2-independent inhibition of APCCdc20 by the mitotic checkpoint protein BubR1. Dev. Cell 1, 227–237 (2001).
Fang, G. Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol. Biol. Cell 13, 755–766 (2002).
Biggins, S. & Murray, A. W. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 15, 3118–3129 (2001).
Tanaka, T. U. et al. Evidence that the Ipl1–Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell 108, 317–329 (2002).
Lampson, M. A., Renduchitala, K., Khodjakov, A. & Kapoor, T. M. Correcting improper chromosome-spindle attachments during cell division. Nature Cell Biol. 6, 232–237 (2004).
Cahill, D. P. et al. Mutations of mitotic checkpoint genes in human cancers. Nature 392, 300–303 (1998).
Shin, H. J. et al. Dual roles of human BubR1, a mitotic checkpoint kinase, in the monitoring of chromosomal instability. Cancer Cell 4, 483–497 (2003).
Shichiri, M., Yoshinaga, K., Hisatomi, H., Sugihara, K. & Hirata, Y. Genetic and epigenetic inactivation of mitotic checkpoint genes hBUB1 and hBUBR1 and their relationship to survival. Cancer Res. 62, 13–17 (2002).
Wang, X. et al. Significance of MAD2 expression to mitotic checkpoint control in ovarian cancer cells. Cancer Res. 62, 1662–1668 (2002).
Hanks, S. et al. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nature Genet. 36, 1159–1161 (2004).
Harrington, E. A. et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nature Med. 10, 262–267 (2004).
Kops, G. J., Foltz, D. R. & Cleveland, D. W. Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint. Proc. Natl Acad. Sci. USA 101, 8699–8704 (2004).
Li, Y. & Benezra, R. Identification of a human mitotic checkpoint gene: hsMAD2. Science 274, 246–248 (1996).
Chan, G. K., Jablonski, S. A., Sudakin, V., Hittle, J. C. & Yen, T. J. Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J. Cell Biol. 146, 941–954 (1999).
Weaver, B. A. et al. Centromere-associated protein-E is essential for the mammalian mitotic checkpoint to prevent aneuploidy due to single chromosome loss. J. Cell Biol. 162, 551–563 (2003).
Rieder, C. L. The structure of the cold-stable kinetochore fiber in metaphase PtK1 cells. Chromosoma 84, 145–158 (1981).
King, J. M., Hays, T. S. & Nicklas, R. B. Dynein is a transient kinetochore component whose binding is regulated by microtubule attachment, not tension. J. Cell Biol. 151, 739–748 (2000).
Waters, J. C., Chen, R. H., Murray, A. W. & Salmon, E. D. Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J. Cell Biol. 141, 1181–1191 (1998).
Ditchfield, C. et al. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161, 267–280 (2003).
Hauf, S. et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 161, 281–294 (2003).
Mayer, T. U. et al. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286, 971–974 (1999).
Andrews, P. D. et al. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell 6, 253–268 (2004).
Zeitlin, S. G., Shelby, R. D. & Sullivan, K. F. CENP-A is phosphorylated by Aurora B kinase and plays an unexpected role in completion of cytokinesis. J. Cell Biol. 155, 1147–1157 (2001).
Kunitoku, N. et al. CENP-A phosphorylation by Aurora-A in prophase is required for enrichment of Aurora-B at inner centromeres and for kinetochore function. Dev. Cell 5, 853–864 (2003).
Earnshaw, W. C. & Rothfield, N. Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma 91, 313–321 (1985).
McEwen, B. F., Heagle, A. B., Cassels, G. O., Buttle, K. F. & Rieder, C. L. Kinetochore fiber maturation in PtK1 cells and its implications for the mechanisms of chromosome congression and anaphase onset. J. Cell Biol. 137, 1567–1580 (1997).
Acknowledgements
We thank A. North and the Rockefeller University Bioimaging facility. We thank D. Compton, W. Dai, T. Yen, C. Walczak, W. Brinkley and D. Allis for gifts of antibodies, and H. Funabiki for discussions. This work was supported by National Institutes of Health grant GM65933 (T.M.K.). M.A.L. is a Goelet fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Fig S1, Fig S2, Fig S3, Fig S4 (PDF 393 kb)
Rights and permissions
About this article
Cite this article
Lampson, M., Kapoor, T. The human mitotic checkpoint protein BubR1 regulates chromosome–spindle attachments. Nat Cell Biol 7, 93–98 (2005). https://doi.org/10.1038/ncb1208
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1208
This article is cited by
-
Physiological relevance of post-translational regulation of the spindle assembly checkpoint protein BubR1
Cell & Bioscience (2021)
-
BUB1B promotes extrahepatic cholangiocarcinoma progression via JNK/c-Jun pathways
Cell Death & Disease (2021)
-
Kinesin-7 CENP-E regulates chromosome alignment and genome stability of spermatogenic cells
Cell Death Discovery (2020)
-
Proper chromosome alignment depends on BRCA2 phosphorylation by PLK1
Nature Communications (2020)
-
CKT0353, a novel microtubule targeting agent, overcomes paclitaxel induced resistance in cancer cells
Investigational New Drugs (2020)