Abstract
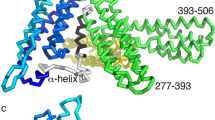
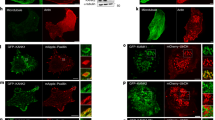
Interactions between microtubule and actin networks are thought to be crucial for mechanical and signalling events at the cell cortex. Cytoplasmic dynein has been proposed to mediate many of these interactions1,2,3. Here, we report that dynein is localized to the cortex at adherens junctions in cultured epithelial cells and that this localization is sensitive to drugs that disrupt the actin cytoskeleton. Dynein is recruited to developing contacts between cells, where it localizes with the junctional proteins β-catenin and E-cadherin. Microtubules project towards these early contacts and we hypothesize that dynein captures and tethers microtubules at these sites. Dynein immunoprecipitates with β-catenin, and biochemical analysis shows that dynein binds directly to β-catenin. Overexpression of β-catenin disrupts the cellular localization of dynein and also dramatically perturbs the organization of the cellular microtubule array. In cells overexpressing β-catenin, the centrosome becomes disorganized and microtubules no longer appear to be anchored at the cortex. These results identify a novel role for cytoplasmic dynein in capturing and tethering microtubules at adherens junctions, thus mediating cross-talk between actin and microtubule networks at the cell cortex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Karki, S. & Holzbaur, E. L. F. Curr. Opin. Cell. Biol. 11, 45–53 (1999).
Goode, B. L., Drubin, D. G. & Barnes, G. Curr. Opin. Cell. Biol. 12, 63–71 (2000).
Fuchs, E. & Karakesisoglou, I. Genes Dev. 15, 1–14 (2001).
Steinberg, M. S. & McNutt, P. M. Curr. Opin. Cell. Biol. 11, 554–560 (1999).
Vasioukhin, V. & Fuchs, E. Curr. Opin. Cell. Biol. 13, 76–84 (2001).
Waterman-Storer, C. M., Salmon, W. C. & Salmon, E. D. Mol. Biol. Cell 11, 2471–2483 (2000).
King, S. M. et al. Biochemistry 37, 15033–15041 (1998).
Tai, A. W., Chuang, J. -Z. & Sung, C.-H. J. Cell Biol. 153, 1499–1509 (2001).
Vasioukhin, V., Bauer, C., Yin, M. & Fuchs, E. Cell 100, 209–219 (2000).
Karki, S., LaMonte, B. & Holzbaur E. L. F. J. Cell Biol. 142, 1023–1034 (1998).
Barth, A. I. M., Pollack, A. L., Altschuler, Y., Mostov, K. E. & Nelson, W. J. J. Cell Biol. 136, 693–706 (1997).
Tokito, M. K., Howland, D. S., Lee, V. M. -Y. & Holzbaur E. L. F. Mol. Biol. Cell 7, 1167–1180 (1996).
Karki, S., Tokito, M. K. & Holzbaur E. L. F. J. Biol. Chem. 272, 5887–5891 (1997).
Huber, A. H., Nelson, W. J. & Weis, W. I. Cell 90, 871–882 (1997).
Acknowledgements
This work was funded by a US NIH grant to E.L.F.H. and by postdoctoral fellowships from the US NIH to L.A.L. and the American Heart Association Southeastern Affiliate to S.K. E.L.F.H. is an Established Investigator of the American Heart Association.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ligon, L., Karki, S., Tokito, M. et al. Dynein binds to β-catenin and may tether microtubules at adherens junctions. Nat Cell Biol 3, 913–917 (2001). https://doi.org/10.1038/ncb1001-913
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1001-913
This article is cited by
-
Microsporidia Have a Peculiar Outer Membrane with Exterior Cytoplasmic Proteins
Acta Parasitologica (2022)
-
Cytoplasmic dynein regulates the subcellular localization of sphingosine kinase 2 to elicit tumor-suppressive functions in glioblastoma
Oncogene (2019)
-
Local inhibition of microtubule dynamics by dynein is required for neuronal cargo distribution
Nature Communications (2017)
-
Quantifying cadherin mechanotransduction machinery assembly/disassembly dynamics using fluorescence covariance analysis
Scientific Reports (2016)
-
Sustained Wnt/β-catenin signalling causes neuroepithelial aberrations through the accumulation of aPKC at the apical pole
Nature Communications (2014)