Abstract
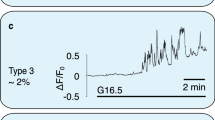
Activation of calcium-ion (Ca2+) channels on the plasma membrane and on intracellular Ca2+ stores, such as the endoplasmic reticulum, generates local transient increases in the cytosolic Ca2+ concentration that induce Ca2+ uptake by neighbouring mitochondria. Here, by using mitochondrially targeted aequorin proteins with different Ca2+ affinities, we show that half of the chromaffin-cell mitochondria exhibit surprisingly rapid millimolar Ca2+ transients upon stimulation of cells with acetylcholine, caffeine or high concentrations of potassium ions. Our results show a tight functional coupling of voltage-dependent Ca2+ channels on the plasma membrane, ryanodine receptors on the endoplasmic reticulum, and mitochondria. Cell stimulation generates localized Ca2+ transients, with Ca2+ concentrations above 20–40 µM, at these functional units. Protonophores abolish mitochondrial Ca2+ uptake and increase stimulated secretion of catecholamines by three- to fivefold. These results indicate that mitochondria modulate secretion by controlling the availability of Ca2+ for exocytosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rizzuto, R., Brini, M., Murgia, M. & Pozzan, T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262, 744–747 (1993).
Rizzuto, R., Bastianutto, C., Brini, M., Murgia, M. & Pozzan, T. Mitochondrial Ca2+ homeostasis in intact cells. J. Cell Biol. 126, 1183–1194 (1994).
Brini, M. et al. Subcellular analysis of Ca2+ homeostasis in primary cultures of skeletal muscle myotubes. Mol. Biol. Cell 8, 129–143 (1997).
Robb-Gaspers, L. D. et al. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 17, 4987–5000 (1998).
Werth, J. L. & Thayer, S. A. Mitochondria buffer physiological calcium loads in cultured rat dorsal root ganglion neurons. J. Neurosci. 14, 346–356 (1994).
White, R. J. & Reynolds, I. J. Mitochondria accumulate Ca2+ following intense glutamate stimulation of cultured rat forebrain neurons. J. Physiol. (Lond.) 498, 31–47 (1997).
Park, Y. B., Herrington, J., Babcock, D. F. & Hille, B. Ca2+ clearance mechanisms in isolated rat adrenal chromaffin cells. J. Physiol. (Lond.) 492, 329–346 (1996).
Herrington, J., Park, Y. B., Babcock, D. F. & Hille, B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron 16, 219–228 (1996).
Babcock, D. F., Herrington, J., Park, Y.-B. & Hille, B. Mitochondrial participation in the intracellular Ca2+ network. J. Cell. Biol. 136, 833–843 (1997).
Xu, T., Naraghi, M., Kang, H. & Neher, E. Kinetic studies of Ca2+ binding and Ca2+ clearance in the cytosol of adrenal chromaffin cells. Biophys. J. 73, 532–545 (1997).
Duchen, M. R. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J. Physiol. (Lond.) 516, 1–17 (1999).
Schinder, A. F., Olson, E. C., Spitzer, N. C. & Montal, M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J. Neurosci. 16, 6125–6133 (1996).
Di Lisa, F. & Bernardi, P. Mitochondrial function as a determinant of recovery or death in cell response to injury. Mol. Cell Biochem. 184, 379–391 (1998).
Green, D. R. & Reed, J. C. Mitochondria and apoptosis. Science 281, 1309–1312 (1998).
Montero, M. et al. Monitoring dynamic changes in free Ca2+ concentration in the endoplasmic reticulum of intact cells. EMBO J. 14, 5467–5475 (1995).
Montero, M., Barrero, M. J. & Alvarez, J. [Ca2+] microdomains control agonist-induced Ca2+ release in intact HeLa cells. FASEB J. 11, 881–885 (1997).
Barrero, M. J., Montero, M. & Alvarez, J. Dynamics of [Ca2+] in the endoplasmic reticulum and cytoplasm of intact HeLa cells. J. Biol. Chem. 272, 27694–27699 (1997).
Montero, M. et al. Ca2+ homeostasis in the endoplasmic reticulum: coexistence of high and low [Ca2+] subcompartments in intact HeLa cells. J. Cell Biol. 139, 601–611 (1997).
Alonso, M. T. et al. Ca2+-induced Ca2+ release in chromaffin cells seen from inside the ER with targeted aequorin. J. Cell Biol. 144, 241–254 (1999).
Rizzuto, R. et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280, 1763–1766 (1998).
Csordás, G., Thomas, A. P. & Hajnóczky, G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 18, 96–108 (1999).
Uceda, G., García, A. G., Guantes, J. M., Michelena, P. & Montiel, C. Effects of Ca2+ channel antagonist subtypes on mitochondrial transport. Eur. J. Pharmacol. 289, 73–80 (1995).
von Rüden, L. & Neher, E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science 262, 1061–1065 (1993).
Neher, E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron 20, 389–399 (1998).
Alonso, M. T. et al. Functional measurements of [Ca2+] in the endoplasmic reticulum using a herpes virus to deliver targeted aequorin. Cell Calcium 24, 87–96 (1998).
Borges, R., Sala, F. & García, A. G. Continuous monitoring of catecholamine release from perfused cat adrenals. J. Neurosci. Meth. 16, 1986).
Acknowledgements
We acknowledge financial support from the Dirección General de Enseñanza Superior (grant PM98/0142 to J.A. and grant PB97/0474 to J.G.-S.), from the Dirección General de Investigación Científica y Técnica (grant PB94/0150) and from Janssen-Cilag to A.G.G., and from Junta de Castilla y León (grant VA19/99 to J.A. and grant VA62/99 to M.T.A.). I.C.-I. and A.A. hold fellowships from the Ministerio de Educación y Ciencia. We thank C. González and T. Pozzan for helpful comments, and J. Fernández for technical assistance.
Correspondence and requests for materials should be addressed to J.A.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Montero, M., Alonso, M., Carnicero, E. et al. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat Cell Biol 2, 57–61 (2000). https://doi.org/10.1038/35000001
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35000001
This article is cited by
-
Mitochondrial Calcium Waves by Electrical Stimulation in Cultured Hippocampal Neurons
Molecular Neurobiology (2023)
-
Curcumin and NCLX inhibitors share anti-tumoral mechanisms in microsatellite-instability-driven colorectal cancer
Cellular and Molecular Life Sciences (2022)
-
Acute reversible SERCA blockade facilitates or blocks exocytosis, respectively in mouse or bovine chromaffin cells
Pflügers Archiv - European Journal of Physiology (2021)
-
Calcium signalling in T cells
Nature Reviews Immunology (2019)
-
Hydrogen sulphide facilitates exocytosis by regulating the handling of intracellular calcium by chromaffin cells
Pflügers Archiv - European Journal of Physiology (2018)