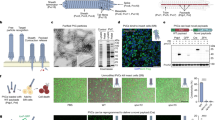
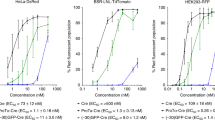
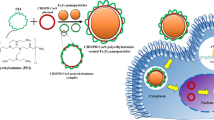
Abstract
Gene delivery has shown potential in a variety of applications, including basic research, therapies for inborn genetic defects, cancer, AIDS, tissue engineering1,2, and vaccination3. Most available systems have serious drawbacks, such as safety hazards4, inefficiency under in vivo–like conditions, and expensive production. When using naked DNA2,5, for instance, a large amount of ultrapure DNA has to be applied as a result of degradation by nucleases6. Similarly, the use of eukaryotic histones7,8, synthetic peptides, or peptide nucleic acids9,10 may be limited by high production costs. We have demonstrated a biotechnologically feasible and economical approach for gene delivery using the histone-like protein from the hyperthermostable eubacterium Thermotoga maritima, TmHU11,12 as an efficient gene transfer reagent. HU can be easily isolated from recombinant Escherichia coli, is extraordinarily stable, and protects dsDNA from thermal denaturation12. This study demonstrates its use as an inexpensive tool for gene delivery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bonadio, J., Smiley, E., Patil, P. & Goldstein, S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat. Med. 5, 753– 759 (1999).
Shea, L.D., Smiley, E., Bonadio, J. & Mooney, D.J. DNA delivery from polymer matrices for tissue engineering. Nat. Biotechnol. 17, 551–554 ( 1999).
Donnelly, J.J., Ulmer, J.B., Shiver, J.W. & Liu, M.A. DNA vaccines. Annu. Rev. Immunol. 15, 617 –648 (1997).
Lehrmann, S. Virus treatment questioned after gene therapy death. Nature 401, 517–518 (1999).
Wolff, J.A. et al. Direct gene transfer into mouse muscle in vivo. Science 247, 1465–1468 (1990).
Barry, M.E. et al. Role of endogenous endonucleases and tissue site in transfection and CpG-mediated immune activation after naked DNA injection. Hum. Gene Ther. 10, 2461–2480 (1999).
Fritz, J.D., Herweijer, H., Zhang, G. & Wolff, J.A. Gene transfer into mammalian cells using histone-condensed plasmid DNA. Hum. Gene Ther. 7, 1395–1404 (1996).
Zaitsev, S.V. et al. H1 and HMG17 extracted from calf thymus nuclei are efficient DNA carriers in gene transfer. Gene Ther. 4, 586–592 (1997).
Subramanian, A., Ranganathan, P. & Diamond, S.L. Nuclear targeting peptide scaffolds for lipofection of nondividing mammalian cells. Nat. Biotechnol. 17, 873–877 ( 1999).
Branden, L.J., Mohamed, A.J. & Smith, C.I.E. A peptide nucleic acid–nuclear localization signal fusion that mediates nuclear transport of DNA. Nat. Biotechnol. 17, 784–787 (1999).
Christodoulou, E. & Vorgias, C.E. Cloning, overproduction, purification and crystallization of the DNA binding protein HU from the hyperthermophilic eubacterium Thermotoga maritima. Acta Crystallogr. D 54, 1043–1045 (1998).
Esser, D., Rudolph, R., Jaenicke, R. & Böhm, G. The HU protein from Thermotoga maritima: recombinant expression, purification and physicochemical characterization of an extremely hyperthermophilic DNA-binding protein. J. Mol. Biol. 291, 1135– 1146 (1999).
Wyman, T.B. et al. Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry 36, 3008–3017 ( 1997).
Nagahara, H. et al. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat. Med. 4, 1449–1452 (1998).
Elliott, G. & O'Hare, P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell 88, 223–233 ( 1997).
Boussif, O., Zanta, M.A. & Behr, J.P. Optimized galenics improve in vitro gene transfer with cationic molecules up to 1000-fold. Gene Ther. 3, 1074–1080 (1996).
Nakai, K. & Kanehisa, M. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics 14, 897–911 ( 1992).
Acknowledgements
Plasmid pEli92 was kindly provided by Ulrich Brinkmann. This work was supported by a grant from Land Sachsen-Anhalt, and D.E. was in part financed by an HSP III grant from the German Academic Exchange Service. This publication is dedicated to Rainer Jaenicke, on the occasion of his 69th birthday.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esser, D., Amanuma, H., Yoshiki, A. et al. A hyperthermostable bacterial histone-like protein as an efficient mediator for transfection of eukaryotic cells. Nat Biotechnol 18, 1211–1213 (2000). https://doi.org/10.1038/81221
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/81221