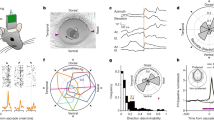
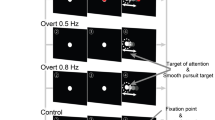
Abstract
Through the development of a high-acuity fovea, primates with frontal eyes have acquired the ability to use binocular eye movements to track small objects moving in space1. The smooth-pursuit system moves both eyes in the same direction to track movement in the frontal plane (frontal pursuit), whereas the vergence system moves left and right eyes in opposite directions to track targets moving towards or away from the observer (vergence tracking). In the cerebral cortex and brainstem, signals related to vergence eye movements—and the retinal disparity and blur signals that elicit them—are coded independently of signals related to frontal pursuit2,3,4,5,6. Here we show that these types of signal are represented in a completely different way in the smooth-pursuit region of the frontal eye fields7,8,9,10,11. Neurons of the frontal eye field modulate strongly during both frontal pursuit and vergence tracking, which results in three-dimensional cartesian representations of eye movements. We propose that the brain creates this distinctly different intermediate representation to allow these neurons to function as part of a system that enables primates to track and manipulate objects moving in three-dimensional space.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Leigh, R. & Zee, D. S. The Neurology of Eye Movements (Oxford Univ. Press, New York, 1999)
Gamlin, P. D. & Yoon, K. An area for vergence eye movement in primate frontal cortex. Nature 407, 1003–1007 (2000)
Cumming, B. in Visual Detection of Motion (eds Smith, A. T. & Snowdon, R. J.) 333–366 (Academic, London, 1994)
Mays, L. E., Porter, J. D., Gamlin, P. D. R. & Tellow, C. A. Neural control of vergence eye movements: neurons encoding vergence velocity. J. Neurophysiol. 56, 1007–1021 (1986)
Mays, L. E. & Gamlin, P. D. Neuronal circuitry controlling the near response. Curr. Opin. Neurobiol. 5, 763–768 (1995)
Zhou, W. & King, W. M. Premotor commands encode monocular eye movements. Nature 393, 692–695 (1998)
MacAvoy, M. G., Gottlieb, J. P. & Bruce, C. J. Smooth pursuit eye movement representation in the primate frontal eye field. Cereb. Cortex 1, 95–102 (1991)
Gottlieb, J. P., MacAvoy, M. G. & Bruce, C. J. Neural responses related to smooth pursuit eye movements and their correspondence with electrically elicited slow eye movements in the primate frontal eye field. J. Neurophysiol. 72, 1634–1653 (1994)
Tian, J. & Lynch, J. C. Functionally defined smooth and saccadic eye movement subregions in the frontal eye field of Cebus monkeys. J. Neurophysiol. 76, 2740–2771 (1996)
Tanaka, K. & Fukushima, K. Neuronal responses related to smooth pursuit eye movements in the periarcuate cortical area of monkeys. J. Neurophysiol. 80, 28–47 (1998)
Fukushima, K., Sato, T., Fukushima, J., Shinmei, Y. & Kaneko, C. R. S. Activity of smooth pursuit-related neurons in the monkey periarcuate cortex during pursuit and passive whole body rotation. J. Neurophysiol. 83, 563–587 (2000)
Maunsell, J. H. & van Essen, D. C. Functional properties of neurons in middle temporal visual area of the macaque monkey. II. Binocular interactions and sensitivity to binocular disparity. J. Neurophysiol. 49, 1148–1167 (1983)
Roy, J. P., Komatsu, H. & Wurtz, R. H. Disparity sensitivity of neurons in monkey extrastriate area MST. J. Neurosci. 12, 2478–2492 (1992)
Eifuku, S. & Wurtz, R. H. Response to motion in extrastriate area MSTl: disparity sensitivity. J. Neurophysiol. 82, 2462–2475 (1999)
Shi, D., Friedman, H. R. & Bruce, C. J. Deficits in smooth pursuit eye movements after muscimol inactivation within the primate frontal eye field. J. Neurophysiol. 80, 458–464 (1998)
Fukushima, K., Sato, T. & Fukushima, J. Vestibular-pursuit interactions: gaze-velocity and target-velocity signals in the monkey frontal eye fields. Ann. NY Acad. Sci. 871, 248–259 (1999)
Ferrera, V. P. & Barborica, A. Predictive responses to invisible target motion in macaque frontal eye field. Soc. Neurosci. Abstr. 26, 669 (2000)
Fukushima, K., Yamanobe, T., Shinmei, Y. & Fukushima, J. Predictive responses of peri-arcuate pursuit neurons to visual target motion. Exp. Brain Res. 145, 104–120 (2002)
Semmlow, J. L., Weihong, Y. & Alvarez, T. L. Evidence for separate control of slow version and vergence eye movements: support for Hering's law. Vision Res. 38, 1145–1152 (1998)
Gamlin, P. D. Subcortical neural circuits for ocular accommodation and vergence in primates. Ophthal. Physiol. Opt. 2, 81–89 (1999)
Judge, S. J. & Cumming, B. G. Neurons in the monkey midbrain with activity related to vergence eye movements and accommodation. J. Neurophysiol. 55, 915–930 (1986)
Takemura, A., Inoue, Y., Kawano, K., Quaia, C. & Miles, F. A. Single-unit activity in cortical area MST associated with disparity-vergence eye movements: evidence for population coding. J. Neurophysiol. 85, 2245–2266 (2001)
Colby, C. L., Duhamel, J. R. & Goldberg, M. E. Ventral intraparietal area of the macaque: anatomic location and visual response properties. J. Neurophysiol. 69, 902–914 (1993)
Ferraina, S., Pare, M. & Wurtz, R. H. Disparity sensitivity of frontal eye field neurons. J. Neurophysiol. 83, 625–629 (2000)
Kakei, S., Hoffman, D. S. & Strick, P. L. Direction of action is represented in the ventral premotor cortex. Nature Neurosci. 4, 1020–1025 (2001)
Mushiake, H., Tanatsugu, Y. & Tanji, J. Neuronal activity in the ventral part of premotor cortex during target-reach movement is modulated by direction of gaze. J. Neurophysiol. 78, 567–571 (1997)
Fogassi, L. et al. Space coding by premotor cortex. Exp. Brain Res. 89, 686–690 (1992)
Fogassi, L. et al. Coding of peripersonal space in inferior premotor cortex (area F4). J. Neurophysiol. 76, 141–157 (1996)
Graziano, M. S., Hu, X. T. & Gross, C. G. Visuospatial properties of ventral premotor cortex. J. Neurophysiol. 77, 2268–2292 (1997)
Krauzlis, R. J. & Lisberger, S. G. Directional organization of eye movement and visual signals in the floccular lobe of the monkey cerebellum. Exp. Brain Res. 109, 289–302 (1996)
Acknowledgements
We thank B. Cumming, M. Goldberg, S. Lisberger, F. Miles and P. Strick for comments on the manuscript, and T. Akao and F. Sato for participation in some experiments. This work was supported in part by CREST of JST, Japanese Ministry of Education, Science, Culture and Sports, and Marna Cosmetics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Fukushima, K., Yamanobe, T., Shinmei, Y. et al. Coding of smooth eye movements in three-dimensional space by frontal cortex. Nature 419, 157–162 (2002). https://doi.org/10.1038/nature00953
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature00953
This article is cited by
-
Oculomotor abnormalities indicate early executive dysfunction in prodromal X-linked dystonia-parkinsonism (XDP)
Journal of Neurology (2023)
-
Three-dimensional binocular eye–hand coordination in normal vision and with simulated visual impairment
Experimental Brain Research (2018)
-
Otolith inputs to pursuit neurons in the frontal eye fields of alert monkeys
Experimental Brain Research (2009)
-
A quantitative synchronization model for smooth pursuit target tracking
Biological Cybernetics (2007)
-
Latency of vestibular responses of pursuit neurons in the caudal frontal eye fields to whole body rotation
Experimental Brain Research (2007)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.