Abstract
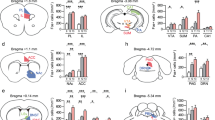
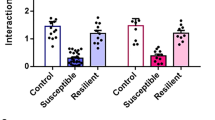
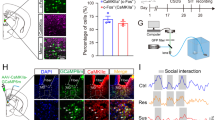
Dopamine in prefrontal cortices is implicated in cognitive and emotional functions, and the dysfunction of prefrontal dopamine has been associated with cognitive and emotional deficits in mental illnesses. These findings have led to clinical trials of dopamine-targeting drugs and brain imaging of dopamine receptors in patients with mental illnesses. Rodent studies have suggested that dopaminergic pathway projecting to the medial prefrontal cortex (mPFC) suppresses stress susceptibility. Although various types of mPFC neurons express several dopamine receptor subtypes, previous studies neither isolated a role of dopamine receptor subtype nor identified the site of its action in mPFC. Using social defeat stress (SDS) in mice, here we identified a role of dopamine D1 receptor subtype in mPFC excitatory neurons in suppressing stress susceptibility. Repeated social defeat stress (R-SDS) reduces the expression of D1 receptor subtype in mPFC of mice susceptible to R-SDS. Knockdown of D1 receptor subtype in whole neuronal populations or excitatory neurons in mPFC facilitates the induction of social avoidance by SDS. Single social defeat stress (S-SDS) induces D1 receptor-mediated extracellular signal-regulated kinase phosphorylation and c-Fos expression in mPFC neurons. Whereas R-SDS reduces dendritic lengths of mPFC layer II/III pyramidal neurons, S-SDS increases arborization and spines of apical dendrites of these neurons in a D1 receptor-dependent manner. Collectively, our findings show that D1 receptor subtype and related signaling in mPFC excitatory neurons mediate acute stress-induced dendritic growth of these neurons and contribute to suppression of stress susceptibility. Therefore, we propose that D1 receptor-mediated dendritic growth in mPFC excitatory neurons suppresses stress susceptibility.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN et al. Mechanisms of stress in the brain. Nat Neurosci 2015; 18: 1353–1363.
Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 2016; 22: 238–249.
Lee RS, Sawa A. Environmental stressors and epigenetic control of the hypothalamic-pituitary-adrenal axis. Neuroendocrinology 2014; 100: 278–287.
Tost H, Champagne FA, Meyer-Lindenberg A. Environmental influence in the brain, human welfare and mental health. Nat Neurosci 2015; 18: 1421–1431.
Tanaka K, Furuyashiki T, Kitaoka S, Senzai Y, Imoto Y, Segi-Nishida E et al. Prostaglandin E2-mediated attenuation of mesocortical dopaminergic pathway is critical for susceptibility to repeated social defeat stress in mice. J Neurosci 2012; 32: 4319–4329.
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 2013; 493: 532–536.
Niwa M, Jaaro-Peled H, Tankou S, Seshadri S, Hikida T, Matsumoto Y et al. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science 2013; 339: 335–339.
Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 2011; 63: 182–217.
Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci 2007; 10: 376–384.
Puig MV, Miller EK. The role of prefrontal dopamine D1 receptors in the neural mechanisms of associative learning. Neuron 2012; 74: 874–886.
Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci 1997; 17: 8528–8535.
Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology 2006; 31: 297–309.
Arnsten AF, Girgis RR, Gray DL, Mailman RB. Novel dopamine therapeutics for cognitive deficits in schizophrenia. Biol Psychiatry 2016; 81: 67–77.
Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature 1997; 385: 634–636.
George MS, Molnar CE, Grenesko EL, Anderson B, Mu Q, Johnson K et al. A single 20 mg dose of dihydrexidine (DAR-0100), a full dopamine D1 agonist, is safe and tolerated in patients with schizophrenia. Schizophr Res 2007; 93: 42–50.
Rosell DR, Zaluda LC, McClure MM, Perez-Rodriguez MM, Strike KS, Barch DM et al. Effects of the D1 dopamine receptor agonist dihydrexidine (DAR-0100A) on working memory in schizotypal personality disorder. Neuropsychopharmacology 2015; 40: 446–453.
Karlsson P, Farde L, Halldin C, Sedvall G. PET study of D(1) dopamine receptor binding in neuroleptic-naive patients with schizophrenia. Am J Psychiatry 2002; 159: 761–767.
Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 2002; 22: 3708–3719.
Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an "inverted-U" toward a family of functions. Front Neurosci 2013; 7: 62.
Santana N, Mengod G, Artigas F. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex 2009; 19: 849–860.
de Almeida J, Mengod G. D2 and D4 dopamine receptor mRNA distribution in pyramidal neurons and GABAergic subpopulations in monkey prefrontal cortex: implications for schizophrenia treatment. Neuroscience 2010; 170: 1133–1139.
Oda S, Funato H, Adachi-Akahane S, Ito M, Okada A, Igarashi H et al. Dopamine D5 receptor immunoreactivity is differentially distributed in GABAergic interneurons and pyramidal cells in the rat medial prefrontal cortex. Brain Res 2010; 1329: 89–102.
Rashid AJ, O'Dowd BF, Verma V, George SR. Neuronal Gq/11-coupled dopamine receptors: an uncharted role for dopamine. Trends Pharmacol Sci 2007; 28: 551–555.
Holmes A, Lachowicz JE, Sibley DR. Phenotypic analysis of dopamine receptor knockout mice; recent insights into the functional specificity of dopamine receptor subtypes. Neuropharmacology 2004; 47: 1117–1134.
Stanwood GD, Parlaman JP, Levitt P. Anatomical abnormalities in dopaminoceptive regions of the cerebral cortex of dopamine D1 receptor mutant mice. J Comp Neurol 2005; 487: 270–282.
Ikegami M, Uemura T, Kishioka A, Sakimura K, Mishina M. Striatal dopamine D1 receptor is essential for contextual fear conditioning. Sci Rep 2014; 4: 3976.
Sarinana J, Kitamura T, Kunzler P, Sultzman L, Tonegawa S. Differential roles of the dopamine 1-class receptors, D1R and D5R, in hippocampal dependent memory. Proc Natl Acad Sci USA 2014; 111: 8245–8250.
Betley JN, Sternson SM. Adeno-associated viral vectors for mapping, monitoring, and manipulating neural circuits. Hum Gene Ther 2011; 22: 669–677.
Golden SA, Covington HE 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc 2011; 6: 1183–1191.
Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007; 131: 391–404.
Lazarus M, Shen HY, Cherasse Y, Qu WM, Huang ZL, Bass CE et al. Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci 2011; 31: 10067–10075.
Hioki H, Kuramoto E, Konno M, Kameda H, Takahashi Y, Nakano T et al. High-level transgene expression in neurons by lentivirus with Tet-Off system. Neurosci Res 2009; 63: 149–154.
Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates, 2nd edn. Academic Press: London, 2001.
Shinohara R, Thumkeo D, Kamijo H, Kaneko N, Sawamoto K, Watanabe K et al. A role for mDia, a Rho-regulated actin nucleator, in tangential migration of interneuron precursors. Nat Neurosci 2012; 15: 373–380, S371–S372.
Ehrlich AT, Furuyashiki T, Kitaoka S, Kakizuka A, Narumiya S. Prostaglandin E receptor EP1 forms a complex with dopamine D1 receptor and directs D1-induced cAMP production to adenylyl cyclase 7 through mobilizing G(betagamma) subunits in human embryonic kidney 293T cells. Mol Pharmacol 2013; 84: 476–486.
Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 1953; 87: 387–406.
Cahill ME, Bagot RC, Gancarz AM, Walker DM, Sun H, Wang ZJ et al. Bidirectional synaptic structural plasticity after chronic cocaine administration occurs through Rap1 small GTPase signaling. Neuron 2016; 89: 566–582.
Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav 1993; 53: 983–993.
Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 2010; 13: 133–140.
Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 2010; 468: 263–269.
Nagai T, Takuma K, Kamei H, Ito Y, Nakamichi N, Ibi D et al. Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn Mem 2007; 14: 117–125.
Xue B, Mao LM, Jin DZ, Wang JQ. Regulation of synaptic MAPK/ERK phosphorylation in the rat striatum and medial prefrontal cortex by dopamine and muscarinic acetylcholine receptors. J Neurosci Res 2015; 93: 1592–1599.
Jungenitz T, Radic T, Jedlicka P, Schwarzacher SW. High-frequency stimulation induces gradual immediate early gene expression in maturing adult-generated hippocampal granule cells. Cereb Cortex 2014; 24: 1845–1857.
Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science 2012; 338: 68–72.
Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci 2012; 15: 689–695.
Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 2006; 26: 7870–7874.
Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science 2009; 325: 621–625.
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 2011; 69: 754–761.
Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci 2001; 2: 880–888.
Harris KM. Structure, development, and plasticity of dendritic spines. Curr Opin Neurobiol 1999; 9: 343–348.
Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience 2006; 139: 263–276.
Takahashi H, Yamada M, Suhara T. Functional significance of central D1 receptors in cognition: beyond working memory. J Cereb Blood Flow Metab 2012; 32: 1248–1258.
Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004; 174: 3–16.
Kosaka J, Takahashi H, Ito H, Takano A, Fujimura Y, Matsumoto R et al. Decreased binding of [11C]NNC112 and [11C]SCH23390 in patients with chronic schizophrenia. Life Sci 2010; 86: 814–818.
Takahashi H. PET neuroimaging of extrastriatal dopamine receptors and prefrontal cortex functions. J Physiol Paris 2013; 107: 503–509.
Dandekar MP, Luse D, Hoffmann C, Cotton P, Peery T, Ruiz C et al. Increased dopamine receptor expression and anti-depressant response following deep brain stimulation of the medial forebrain bundle. J Affect Disord 2017; 217: 80–88.
Pei L, Li S, Wang M, Diwan M, Anisman H, Fletcher PJ et al. Uncoupling the dopamine D1-D2 receptor complex exerts antidepressant-like effects. Nat Med 2010; 16: 1393–1395.
Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 2004; 51: 32–58.
Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 2012; 492: 428–432.
Christoffel DJ, Golden SA, Walsh JJ, Guise KG, Heshmati M, Friedman AK et al. Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nat Neurosci 2015; 18: 962–964.
Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex 2009; 19: 2479–2484.
Gonzalez-Islas C, Hablitz JJ. Dopamine enhances EPSCs in layer II-III pyramidal neurons in rat prefrontal cortex. J Neurosci 2003; 23: 867–875.
Cline HT, Constantine-Paton M. NMDA receptor antagonists disrupt the retinotectal topographic map. Neuron 1989; 3: 413–426.
Sin WC, Haas K, Ruthazer ES, Cline HT. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature 2002; 419: 475–480.
English JD, Sweatt JD. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J Biol Chem 1996; 271: 24329–24332.
Wu GY, Deisseroth K, Tsien RW. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat Neurosci 2001; 4: 151–158.
Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V et al. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron 2006; 50: 897–909.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959–964.
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011; 475: 91–95.
Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science 2000; 287: 2020–2022.
Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry 2000; 47: 305–313.
Pfleiderer B, Michael N, Erfurth A, Ohrmann P, Hohmann U, Wolgast M et al. Effective electroconvulsive therapy reverses glutamate/glutamine deficit in the left anterior cingulum of unipolar depressed patients. Psychiatry Res 2003; 122: 185–192.
Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA 2008; 105: 751–756.
Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 2008; 13: 717–728.
Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT et al. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci 2011; 31: 6277–6288.
Acknowledgments
We thank Dr Dai Watanabe for his helpful comments and discussions; Tae Arai, Akiko Washimi, Yuki Nakanishi and Misako Takizawa for secretarial helps; and Nodoka Asamoto for animal breeding and care. The present study was supported by a grant from AMED-CREST of JST (to SN), Grants-in-Aids for Scientific Research from the Japan Society for the Promotion of Science (24689015 and 16H05132 to TF; 13J04246 and 16K18365 to RS), Grants-in-Aids for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan (25116517, 25116715, 15H01289, 17H05572 and 17H06057 to TF), a grant from AMED (to TF), grants from the Uehara Memorial Foundation (to TF), the Sumitomo Foundation (to TF), the Astellas Foundation for Research on Metabolic Disorders (to TF) and the Takeda Science Foundation (to TF), United States Public Health Service Grants MH-084018, MH-094268, MH-069853, MH-085226, MH-088753 and MH-092443 (to AS) and grants from Stanley (to AS), RUSK (to AS), S-R foundations (to AS), NARSAD (to AS) and Maryland Stem Cell Research Fund (to AS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Shinohara, R., Taniguchi, M., Ehrlich, A.T. et al. Dopamine D1 receptor subtype mediates acute stress-induced dendritic growth in excitatory neurons of the medial prefrontal cortex and contributes to suppression of stress susceptibility in mice. Mol Psychiatry 23, 1717–1730 (2018). https://doi.org/10.1038/mp.2017.177
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.177
This article is cited by
-
Sex Differences in Rats with the Valproate Model of Autism: Disturbances in Social Behavior and Changes in Drd1 Gene Expression in Various Brain Structures
Neuroscience and Behavioral Physiology (2023)
-
Treadmill Exercise Improves Behavioral and Neurobiological Alterations in Restraint-Stressed Rats
Journal of Molecular Neuroscience (2023)
-
Structure and function differences in the prelimbic cortex to basolateral amygdala circuit mediate trait vulnerability in a novel model of acute social defeat stress in male mice
Neuropsychopharmacology (2022)
-
Chronic social defeat stress increases the amounts of 12-lipoxygenase lipid metabolites in the nucleus accumbens of stress-resilient mice
Scientific Reports (2022)
-
Effects of Acute Swimming Stress on the Behavioral and Neurochemical Effects of Pyrazolo[C]pyridine Derivative GIZh-72 and Diazepam in BALB/c and C57BL/6 Mice
Neuroscience and Behavioral Physiology (2022)