Abstract
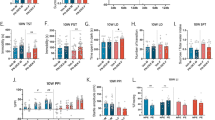
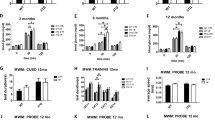
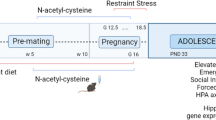
Dysregulation of stress hormones, such as glucocorticoids, in adult life increases the risk to develop Alzheimer’s disease (AD). However, the effect of prenatal glucocorticoids exposure on AD development in the offspring remains unknown. We studied how gestational dexamethasone exposure influences the AD-like phenotype in the offspring of triple transgenic AD mice (3 × Tg). To this end, female mice received dexamethasone or vehicle during the entire pregnancy time in the drinking water. Offspring from vehicle-treated 3 × Tg (controls) were compared with offspring from dexamethasone-treated 3 × Tg later in life for their memory, learning ability and brain pathology. Compared with controls, offspring from dexamethasone-treated mothers displayed improvement in their memory as assessed by fear conditioning test, both in the cue and recall phases. The same animals had a significant reduction in the insoluble fraction of tau, which was associated with an increase in autophagy. In addition, they showed an activation of the transcription factor cellular response element-binding protein and an increase in brain-derived neurotrophic factor and c-FOS protein levels, key regulators of synaptic plasticity and memory. We conclude that dexamethasone exposure during pregnancy provides long-lasting protection against the onset and development of the AD-like phenotype by improving cognition and tau pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA . Chronic psychological distress and risk of Alzheimer's disease in old age. Neuroepidemiology 2006; 27: 143–153.
Wilson RS, Begeny CT, Boyle PA, Scneider JA, Bennett DA . Vulnerability to stress, anxiety, and development of dementia in old age. Am J Geriatr Psychiatry 2011; 19: 327–334.
Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM et al. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry 2006; 163: 2164–2169.
Huang CW, Lui CC, Chang WN, Lu CH, Wang YL, Chang CC . Elevated basal cortisol level predicts lower hippocampal volume and cognitive decline in Alzheimer’s disease. J Clin Neurosci 2009; 16: 1283–1286.
Aznar S, Knudsen GM . Depression and Alzheimer’s disease: is stress the initiating factor in a common neuropathological cascade. J Alzheimer’s Dis 2011; 23: 177–193.
Baglietto-Vargas D, Medeiros R, Martinez-Coria H, LaFerla FM, Green KM . Mifepristone alters amyloid precursor protein processing to preclude amyloid beta and tau pathology. Biol Psychiatry 2013; 74: 357–366.
Lee KW, Kim JB, Seo KS, Kim TK, Im JY, Baek IS et al. Behavioral stress accelerates plaque pathogenesis in the brain of Tg2576 mice via generation of metabolic oxidative stress. J Neurochem 2009; 108: 165–175.
Mueller BR, Bale TL . Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav 2007; 91: 55–65.
Son GH, Geum D, Chung S, Kim EJ, Jo JH, Kim CM et al. Maternal stress produces learning deficits associated with impairment of NMDA receptor-mediated synaptic plasticity. J Neurosci 2006; 26: 3309–3318.
Joshi YB, Praticò D . The involvement of 5-lipoxygenase activating protein in anxiety-like behavior. J Psychiatr Res 2013; 47: 694–698.
Chu J, Li JG, Praticò D . Zileuton improves memory deficits, amyloid and tau pathology in a mouse model of Alzheimer’s disease with plaques and tangles. PLoS One 2013; 8: e70991.
Chu J, Praticò D . 5Lipoxygenase as an endogenous modulator of amyloid beta formation in vivo. Ann Neurol 2011; 69: 34–46.
Joshi YB, Chu J, Praticò D . Knockout of 5-lipoxygenase prevents dexamethasone-induced tau pathology in 3xTg mice. Aging Cell 2013; 12: 706–711.
Shinohara M, Fujioka S, Murray ME, Wojtas A, Baker M, Rovelet-Lecrux A et al. Regional distribution of synaptic markers and APP correlate with distinct clinicopathological features in sporadic and familial Alzheimer's disease. Brain 2014; 137: 1533–1549.
Finsterwald C, Alberini CM . Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies. Neurobiol Learn Mem 2014; 112C: 17–29.
Zhang X, Li L, Zang X, Xie W, Li L, Yang D et al. Prenatal hypoxia may aggravate the cognitive impairment and Alzheimer’s disease neuropathology in APPswe/PS1A246E transgenic mice. Neurobiol Aging 2013; 34: 663–678.
Mazumdar M, Xia W, Hofmann O, Gregas M, Ho Sui S, Hide W et al. Prenatal lead levels, plasma amyloid beta levels, and gene expression in young adulthood. Environ Health Perspect 2012; 120: 702–707.
Green KN, Bilings LM, Roozendaal B, Mc Gaugh JL, LaFerla FM . Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci 2006; 26: 9047–9056.
Seckl JR . Prenatal glucocorticoids and long-term programming. Eur J Endocrinol 2004; 151: U49–U62.
Riedmann T, Patchev AV, Cho K, Almeida OFX . Corticosteroids: way upstream. Mol Brain 2010; 3: 2.
Newnham JP, Moss TJ . Antenatal glucocorticoids and growth: single versus multiple doses in animal and human studies. Sem Neonatol 2001; 6: 285–292.
Bloom SL, Sheffield JS, McIntire DD, Leveno KJ . Antenatal dexamethasone and decreased birth weight. Obstr Gynecol 1999; 97: 485–490.
Mercado AB, Wilson RC, Cheng KC, Wei JQ . New MI. Prenatal treatment and diagnosis of congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. J Clin Endocr Metabol 1995; 80: 2014–2020.
Weinstock M . The long-term behavioral consequences of prenatal stress. Neurosci Biobehav Rev 2008; 32: 1073–1086.
Brunton PJ . Effects of maternal exposure to social stress during pregnancy: consequences for mother and offspring. Reproduction 2013; 146: R175–R189.
Frodl T, O’Keane V . How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis 2013; 52: 24–37.
Lemaire V, Koehl M, Le Moal M, Abrous DN . Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA 2000; 97: 11032–11037.
Vallee M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S . Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. J Neurosci 1997; 17: 2626–2636.
Fujioka T, Fujioka A, Tan N, Chowdhury GM, Mouri H, Sakata Y et al. Mild prenatal stress enhances learning performances in the non-adopted rat offspring. Neuroscience 2001; 103: 301–307.
Herring A, Donath A, Yarmolenko M, Uslar E, Conzen C, Kanakis D et al. Exercise during pregnancy mitigates Alzheimer-like pathology in mouse offspring. FASEB J 2012; 26: 117–128.
Carroll JC, Iba M, Bangasser DA, Valentine RJ, James MJ, Bruden KM et al. Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. J Neurosci 2011; 31: 14436–14449.
Joshi YB, Chu J, Praticò D . Stress hormone leads to memory deficits and altered tau phosphorylation in a model of Alzheimer’s disease. J Alzheimers Dis 2012; 31: 167–176.
Sierksma AS, Vanmierlo T, De Vry J, Raijmakers ME, Steinbusch HW, van den Hove DL et al. Effects of prenatal stress exposure on soluble Abeta and brain-derived neurotrophic factor signaling in male and female APPswe/PS1dE9 mice. Neurochem Int 2012; 61: 697–701.
Sierksma AS, Prickaerts J, Chouliara S, Rostamian S, Delbroek L, Rutten BP et al. Behavioral and neurobiological effects of prenatal stress exposure in male and female APPswe/PS1dE9 mice. Neurobiol Aging 2013; 34: 319–337.
Yehuda R, Southwick SR, Krystal JH, Bremner D, Charney DS, Mason JW . Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry 1993; 150: 83–86.
Hougaard KS, Andersen MB, Kjaer SL, Hansen AM, Werge T, Lund SP . Prenatal stress may increase vulnerability to life events: comparison with the effects of prenatal dexamethasone. Brain Res Dev Brain Res 2005; 159: 55–63.
Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE . The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn 2007; 65: 209–237.
Nagano M, Ozawab H, Suzuki H . Prenatal dexamethasone exposure affects anxiety-like behaviour and neuroendocrine systems in an age-dependent manner. Neurosci Res 2008; 60: 364–371.
Roozendaal B, McEwen BS, Chattarji S . Stress memory and the amygdala. Nat Rev Neurosci 2009; 10: 423–433.
Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ et al. Correlation of Alzheimer disease neuropathological changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 2012; 71: 362–381.
Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB: CBP-dependent transcriptional activation. J Neurosci 2007; 27: 6128–6140.
Wood MA, Attner MA, Oliveira AMM, Brindle PK, Abel T . A transcription factor-binding domain of the co-activator CBP is essential for long-term memory and the expression of specific target genes. Learn Mem 2006; 13: 609–617.
Caccamo A, Maldonado MA, Bokov AF, Majumder S, Oddo S . CBP gene transfer increases BDNF levels and ameliorates learning and memory deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 2010; 107: 22687–22692.
Bingham BC, Sheela Rani CS, Frazer A, Strong R, Morilak DA . Exogenous prenatal corticosterone exposure mimics the effects of prenatal stress on adult brain stress response systems and fear extinction behavior. Psychoneuroendocrinology 2013; 38: 2746–2757.
Makoto M, Kiyofumi Y, Naoya M, Kuniaki S, Mitsuru S, Toshitaka N . CREB phosphorylation as a molecular marker of memory processing in the hippocampus for spatial learning. Behav Brain Res 2002; 133: 135–141.
Acknowledgements
This study was in part supported by a grant from the Alzheimer Art Quilt Initiative. We thank Dr Peter Davies for kindly providing the PHF-1 antibody.
Author Contributions
DMA and JYB designed the study and interpreted the data under the supervision of DP. DMA, JYB and EL executed all the experiments. DMA and DP wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Di Meco, A., Joshi, Y., Lauretti, E. et al. Maternal dexamethasone exposure ameliorates cognition and tau pathology in the offspring of triple transgenic AD mice. Mol Psychiatry 21, 403–410 (2016). https://doi.org/10.1038/mp.2015.78
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.78
This article is cited by
-
Differential effects of chronic immunosuppression on behavioral, epigenetic, and Alzheimer’s disease-associated markers in 3xTg-AD mice
Alzheimer's Research & Therapy (2021)
-
Downregulation of autophagy by 12/15Lipoxygenase worsens the phenotype of an Alzheimer’s disease mouse model with plaques, tangles, and memory impairments
Molecular Psychiatry (2021)
-
A pharmacological chaperone improves memory by reducing Aβ and tau neuropathology in a mouse model with plaques and tangles
Molecular Neurodegeneration (2020)
-
RETRACTED ARTICLE: Full recovery of the Alzheimer’s disease phenotype by gain of function of vacuolar protein sorting 35
Molecular Psychiatry (2020)