Abstract
Ovarian low-grade serous carcinomas are thought to evolve in a stepwise fashion from ovarian epithelial inclusions, cystadenomas, and borderline tumors. The current study was designed to gain insight into the origins of low-grade serous carcinomas (tubal versus ovarian) by comparatively evaluating the morphologic (secretory and ciliated cell distribution) and immunophenotypic (using antibodies to PAX8, tubulin, calretinin, and Ki67) attributes of its putative precursor lesions, the normal tubal epithelium, and the overt malignancy. A total of 226 adnexal tissues from 178 patients were studied, including 98 adnexae removed for non-neoplastic indications, 48 serous cystadenomas, 42 serous borderline tumors, and 38 low-grade serous carcinomas. Normal distal tubal epithelium comprised an admixture of PAX8+/tubulin− secretory cells and PAX8−/tubulin+ ciliated cells with a proliferative index of ∼3%. The vast majority of ovarian surface epithelia displayed a mesothelial phenotype (calretinin+/PAX8−/tubulin−) and low proliferative index (0% (12 per 1000)), although 4% of cases also displayed foci with tubal phenotype (calretinin−/PAX8+/tubulin+). In contrast, most (78%) of the ovarian epithelial inclusions displayed a tubal phenotype and had a significantly higher proliferative index (1%) than ovarian surface epithelium, indicating that in most cases, the ovarian surface epithelium and ovarian epithelial inclusions are of different lineages. There was a progressive decrease in the population of ciliated cells, as evidenced by increasing secretory/ciliated cell ratio, from ovarian epithelial inclusions/cystadenomas to borderline tumors to low-grade serous carcinoma, indicating that the latter is a clonal expansion of secretory cells. Overall, the findings make a strong argument that the ovarian epithelial inclusions with a tubal phenotype is likely derived from fallopian tube through an intraovarian endosalpingiosis rather than through Mullerian metaplasia from ovarian surface epithelium. Genetic and molecular studies are needed to further confirm this finding as tubal origination of ovarian serous cancers will have a significant impact on ovarian cancer prevention and management.
Similar content being viewed by others
Main
Until recently, the incessant ovulation theory put forward by Fathalla1 in 1971 has been the most widely accepted theory of ovarian carcinogenesis. According to this theory, constant ovulation-induced damage and repair of the ovarian surface epithelium eventually results in its malignant transformation to ovarian epithelial cancers.2 However, several morphologic, epidemiologic, and molecular observations have gradually accumulated over the ensuing decades such that, at present time, the ovarian surface epithelium is not considered to be the cell of origin of ovarian epithelial cancers by most investigators. The reasons include different histologic and immunophenotypes between ovarian surface epithelium and ovarian epithelial cancers, the pathologic properties of ovarian epithelial cancers showing striking similarities to Mullerian epithelia,3 and the presence of precancerous lesions of ovarian high-grade serous carcinomas in tubal fimbria but not in the ovary or on ovarian surface.4, 5, 6, 7, 8, 9 All these findings, as well as other factors, have led to an increased recognition in recent years that the fallopian tube is the likely source of most pelvic high-grade serous carcinomas.6, 10
Recent molecular studies have suggested that ovarian epithelial cancers can be divided into two broad groups.11 In this model, high-grade serous carcinoma, which constitute the majority of pelvic carcinomas, are classified as type II. Type II cancers commonly show genetic instability, TP53 mutations, and are usually not found in association with precursor lesions within the ovary.12 The ‘ovarian’ serous carcinomas that are putatively derived from distal fallopian tube are predominantly high grade.13, 14, 15, 16 In contrast, the less common low-grade serous carcinoma is classified as a type I cancer in this model. Low-grade serous carcinoma displays more genetic stability, shows specific mutations in genes such as BRAF and KRAS, and seems to evolve in a stepwise fashion from ovarian epithelial inclusions, benign cystadenomas, and borderline tumors.11 Accordingly, in many current models of pelvic serous carcinogenesis, the majority of pelvic high-grade serous carcinomas originate from the endosalpinx, whereas most low-grade serous carcinomas originate in the ovary from ovarian epithelial inclusions. The cell of origin of ‘ovarian’ low-grade serous carcinoma is therefore best evaluated by analyzing the earliest putative precursor, the ovarian epithelial inclusions.
In the current study, a detailed comparative morphologic and immunophenotypic evaluation of low-grade serous carcinoma and its putative precursors was performed in order to gain some insights into the origins of the cancer (fallopian tube versus ovary).
Materials and methods
Case Selection
Slides of adnexal tissues, all resected between 2000 and 2010 from a total of 178 patients, were used in this study. All were obtained from the archived files of the pathology department at the University of Arizona College of Medicine following approval from its institutional review board. The first group comprised 128 adnexal serous tumors, including serous cystadenomas (n=48), serous borderline tumors (n=42), and low-grade serous carcinomas (n=38), that had all previously been categorized as being of ovarian origin based on conventional criteria, that is, predominant localization of disease. These cases will hereafter be referred to as ‘ovarian’. The diagnosis of the ovarian tumors was typically established by the morphologic examination only, with rare cases requiring immunohistochemical confirmation. The histologic diagnoses were verified by concurrent pathologic review by two co-authors (NA and WZ) using a variation on published criteria.17 The second group consisted of 98 ovaries and fallopian tubes from 50 patients who had previously undergone a total hysterectomy and bilateral salpingo-oophorectomy for uterine diseases or for adnexal pathologic changes that were ultimately deemed to be non-neoplastic. Patients’ age in the second group was matched to the first group. All other relevant clinical information was extracted from the pathology reports and clinical records as necessary.
Morphologic Analysis
Among the 98 ovaries, 48 showing ovarian surface epithelium covering at least 10% of the ovarian surface and at least 1 ovarian epithelial inclusions in a single tissue section were studied. The number of ovarian epithelial inclusions in each section (case) was counted. The morphologic features of ovarian surface epithelium and ovarian epithelial inclusions (columnar versus attenuated/low cuboidal) were documented for each case. The number of secretory and ciliated cells within the tubal fimbria epithelium was evaluated by light microscopy.
Immunohistochemical Analyses
Regular immunohistochemical method
Immunohistochemical studies, using antibodies to PAX8, calretinin, tubulin, and MIB1, were performed. PAX8 is a member of the PAX gene family, consisting of nine well-described transcription factors (PAX 1–9).18 PAX8 expression is present in the secretory, but not ciliated epithelial cell population of the normal human fallopian tube,19 and may play a role in differential diagnosis of ovarian epithelial cancers from histologic mimics.19, 20, 21, 22, 23 Calretinin is a recognized mesothelial cell marker, which has been successfully applied to differentiate ovarian serous carcinomas from mesotheliomas, although a few ovarian high-grade serous carcinomas can have weak and focal positivity.24, 25, 26 Tubulin identifies cellular surface cilia, and is therefore an appropriate marker to identify ciliated cells.27 The MIB1 antibody is directed against the proliferation marker Ki-67, which is present in the G1, S, M, and G2 phases of the cell cycle. Proliferative endometrial tissue sections served as positive controls for PAX8 and MIB1 staining, fallopian tube sections for tubulin, and normal mesothelium for calretinin. Negative controls were carried out by replacing primary antibodies with class-matched mouse immunoglobulin G on parallel sections. The subcellular staining localization is nuclear for PAX8 and MIB1, cytoplasmic for calretinin, and apical/glycocalyceal for tubulin.
Dual immunohistochemical staining
To better highlight the different cell populations within the ovarian epithelial inclusions, especially as the lining cells were occasionally morphologically indifferent, we performed dual immunohistochemical staining with both PAX8 and calretinin in representative ovarian epithelial inclusion sections. The dual-staining procedure has previously been described elsewhere.28 The specific signal for PAX8 was visualized by incubation with peroxidase followed by incubation with Diaminobenzidine as chromogen creating a brown nuclear staining product. Then, calretinin immunohistochemical was performed by using alkaline phosphatase generating a bright red product in cytoplasm.
Scoring
To potentially gain some insight into the histogenesis of the ovarian tumors based on the differential distribution (qualitative and quantitative) of secretory cells and ciliated cells in the ovaries (ovarian surface epithelium and ovarian epithelial inclusions) and fallopian tubes, the secretory-to-ciliated cell ratios in these tissues were determined. Ciliated cells were counted using routine light microscopy, by counting at least 500 cells or all available cells if the total number of cells was <500. The secretory-to-ciliated cell ratio was determined for selected ovarian epithelial inclusions in each case (see below). Immunohistochemical determinations of the secretory-to-ciliated cell ratio were based on the PAX8- positive/tubulin-positive ratio in a representative focus. The proliferative index in each case was determined by selecting the most proliferative region in the slide of interest, selecting a microscopic field, counting the number of cells displaying nuclear MIB1 positivity and the number of cells in the background, and extrapolating the findings to the whole slide.
Statistical Analysis
The data were analyzed by standard contingency table methods and nonparametric Mann–Whitney U-tests using the Eproliferative index LOG (Epicenter Software, Pasadena, CA, USA) and Stat View (SAS Institute, Cary, NC, USA) computer package programs. Fisher's exact tests were used to calculate two-sided P-values.
Results
The patients in this study ranged in age from 30 to 72 years (mean 49.5 years). All were without a known family history of breast cancer, ovarian cancers, or BRCA mutations.
Biomarker Expression and Cell Composition in Tube Fimbria
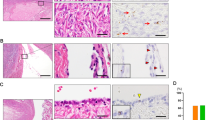
Sections of the fimbriated ends of a total of 50 fallopian tubes were studied immunohistochemically using PAX8, tubulin, calretinin, and MIB1 antibodies. All tubal secretory cells were positive for PAX8, but negative for tubulin, whereas the tubal ciliated cells showed the opposite (Figure 1). All tubal cells were negative for calretinin. The number of tubulin-positive ciliated cells ranged from 34 to 77%, with an average of 65%. MIB1 immunohistochemical stain was done in 42 tubal fimbriated sections, and an average of 3% of the epithelial cells was positive in nuclear pattern. The comparison of cellular proliferative activity between tubal epithelium and ovarian epithelial inclusions is described below.
Immunophenotype of tubal fimbria and ovarian epithelial inclusions. Top panel shows morphologic appearance of tubal fimbria epithelia, mesothelium-derived ovarian epithelial inclusions, and fallopian tube-derived ovarian epithelial inclusions, followed by immunohistochemical stainings of PAX8, calretinin, and tubulin, respectively. PAX8 (nuclear) and tubulin (apical border) staining was seen in tube and fallopian tube-derived ovarian epithelial inclusions, but not in mesothelium-derived ovarian epithelial inclusions. Conversely, calretinin was positive in mesothelium-derived ovarian epithelial inclusions and negative in tube and fallopian tube-derived ovarian epithelial inclusions (original magnification: left panels × 200).
Morphologic and Immunophenotype of Ovarian Surface Epithelia
As previously noted, 48 ovaries showing ovarian surface epithelium covering at least 10% of the ovarian surface were studied. Microscopically, the ovarian surface epithelium predominantly comprised uniform attenuated (flattened), low cuboidal cells. However, 2 (4%) of 48 ovarian sections contained a columnar type of ovarian surface epithelium. Admixed within these columnar-appearing epithelial cells was a minority population of ciliated cells. Immunohistochemical studies for calretinin, PAX8, and tubulin were performed on all ovarian sections, and the results largely mirrored the aforementioned morphologic findings. The cases could be classified into two distinct groups by immunophenotype. The great majority (46 (96%) of 48 cases) were lined only by typical calretinin-positive PAX8-negative (calretinin+/PAX8−) ovarian surface epithelium, whereas the remaining 2 cases displayed ovarian surface epithelium with calretinin+/PAX8− as well as areas of calretinin−/PAX8+ immunophenotype. For descriptive purposes, ovarian surface epithelium with mesothelial phenotype (calretinin+/PAX8−) was designated as mesothelium-derived ovarian surface epithelium, whereas with fallopian tubal phenotype (calretinin−/PAX8+) as fallopian tube-derived ovarian surface epithelium (Figure 2). Mesothelium-derived ovarian surface epithelium was present in all ovarian sections examined. Ciliated cells were not found in mesothelium-derived ovarian surface epithelium, whereas they were identified in the fallopian tube-derived ovarian surface epithelium. The cell proliferative index (MIB1) was 2.5% in fallopian tube-derived ovarian surface epithelial cells, which was significantly higher than the proliferative index in mesothelium-derived ovarian surface epithelial cells (0% (12 per 1000); Table 1). Compared with the immunophenotype of tubal fimbria, fallopian tube-derived ovarian surface epithelium was largely similar, whereas mesothelium-derived ovarian surface epithelium was entirely different.
Ovarian surface epithelia with two different immunophenotypes. One comprised flattened, calretinin-positive, PAX8-negative cells with no significant proliferative activity (negative MIB1), consistent with a mesothelium derivation (right side of the panel, mesothelium-derived ovarian surface epithelium), whereas the other comprised columnar, calretinin-negative, PAX8-positive secretory cells with a high number of MIB1-positive cells, consistent with tubal phenotype (left side of the panel, fallopian tube-derived ovarian surface epithelium). These two types of ovarian surface epithelia showed no gradual transitions or junctional zone in between (original magnification: left panels × 200).
Morphology and Immunophenotype of Ovarian Epithelial Inclusions
Ovarian epithelial inclusions have traditionally been thought to be formed via an invagination of ovarian surface epithelium into ovarian cortex, with malignant transformation of the ensuing cystic lining, possibly after Mullerian metaplasia, forming the histogenetic basis for ovarian serous cancers.29 We sought to determine whether the morphologic and immunophenotypic attributes of ovarian epithelial inclusions are consistent with an ovarian surface epithelium derivation or a tubal derivation.
A total of 856 ovarian epithelial inclusions were identified in sections of 45 ovaries. The number of ovarian epithelial inclusions ranged from 1 to 103 with an average of 19 per ovarian section. Cytologically, there were mainly two types of ovarian epithelial inclusions identified. The majority of ovarian epithelial inclusions contained secretory and ciliated cells, as is characteristic of tubal epithelium, whereas the others were lined by flattened indifferent type of cells without cilia, which are identical to mesothelium-derived ovarian surface epithelium. The majority of ovarian epithelial inclusions were <2 mm. When ovarian epithelial inclusions were >2 mm in diameter, they were lined predominantly by flattened cells. Occasionally, ovarian epithelial inclusions with ciliated and secretory cells formed papillae. No cytologic atypia was identified in all ovarian epithelial inclusions epithelial cells. Stromal cells adjacent to ovarian epithelial inclusions were mainly ovarian stromal cells.
Similar to ovarian surface epithelium, there were two groups of immunophenotypically distinct ovarian epithelial inclusions: one with tubal phenotype (calretinin−/PAX8+) was defined as fallopian tube-derived ovarian epithelial inclusions and the other with mesothelial phenotype (calretinin+/PAX8−) as mesothelium-derived ovarian epithelial inclusions. Of 856 ovarian epithelial inclusions, 667 (78%) were fallopian tube-derived ovarian epithelial inclusions, whereas only 188 (22%) were mesothelium-derived ovarian epithelial inclusions. Therefore, fallopian tube-derived ovarian epithelial inclusions was 3.54 times more common in the ovarian cortex than mesothelium-derived ovarian epithelial inclusions (P<0.001). Ciliated cells were seen in all fallopian tube-derived ovarian epithelial inclusions, and none were clearly discernable in the mesothelium-derived ovarian epithelial inclusions. A total of 400 randomly selected ovarian epithelial inclusions from 20 ovaries were selected for additional microscopic analysis: the number of the ciliated cells ranged from 1 to 35 (average of 12 per fallopian tube-derived ovarian epithelial inclusions). However, analysis of the corresponding tubulin-stained sections showed a higher number of ciliated cells (average of 18 per fallopian tube-derived ovarian epithelial inclusions), indicative of a greater sensitivity of immunohistochemical stains in identifying ciliated cells when compared with morphologic observation. Overall, the morphologic and immunophenotypic attributes of the fallopian tube-derived ovarian epithelial inclusions were very similar to those of the tubal fimbriated end epithelium, and were notably different from those of the mesothelium-derived ovarian surface epithelium. The converse was true of mesothelium-derived ovarian epithelial inclusions, which was more comparable with mesothelium-derived ovarian surface epithelium than tubal epithelium (Table 2 and Figure 1).
To further confirm that two types of ovarian epithelial inclusions were present in ovarian cortex, we performed dual immunohistochemical staining with PAX8 and calretinin in the aforementioned 20 ovarian sections (Figure 3).
Ovarian epithelial inclusions with double PAX8 and calretinin staining. Ovarian epithelial inclusions were occasionally morphologically indifferent (left panel). However, double staining (right panel) with both calretinin (red) and PAX8 (brown) showed that these ovarian epithelial inclusions had discrepant immunostaining patterns. Fallopian tube-derived ovarian epithelial inclusions (PAX8+/calretinin−) are on the top right panel and mesothelium-derived ovarian epithelial inclusions (PAX8−/calretinin+) in the middle right, whereas the panel in the low right shows one large fallopian tube-derived ovarian epithelial inclusion (middle) and one small mesothelium-derived ovarian epithelial inclusion (upper right). The ovarian surface epithelia on the top right panel show PAX8−/calretinin+, indicative of mesothelial origin. A few calretinin+/PAX8− cells seen in the low right (low right panel) represent luteinized ovarian stromal cells (original magnification: × 100).
Immunophenotypes of Ovarian Serous Tumors
All serous tumors, including cystadenomas (n=48), borderline tumors (n=42), and low-grade serous carcinomas (n=28), showed a positive PAX8 and negative calretinin immunophenotype (Figure 4). Tubulin-positive ciliated cells were present in all benign and borderline tumors but were essentially absent in low-grade serous carcinoma cases (Figure 4). There was a trend toward progressive loss of tubulin expression from serous cystadenomas to serous borderline tumors. As expected, the reverse trend was seen regarding MIB1 proliferative indices, which was significantly increased from cystadenomas (5±2%) to borderline tumors (15±5%), and to low-grade serous carcinomas (32±12%).
Secretory cell expansion in the process of low-grade serous carcinoma development. The left panels (a, c, e and g) show PAX8+ secretory cells in fallopian tube-derived ovarian epithelial inclusions, serous cystadenoma, serous borderline tumor, and low-grade serous carcinoma. The right panels (b, d, f and h) show tubulin stain that highlights cilia on cell apical border. Tubulin-positive cilia were present in ∼30% of the fallopian tube-derived ovarian epithelial inclusions cells, 10% of benign cystadenoma cells (arrow), <5% of borderline tumor cells (arrow), and none in low-grade serous carcinoma cells.
Cellular Proliferative Activity in Ovarian Surface Epithelium, Ovarian Epithelial Inclusions, and Tubal Epithelia
To quantitate the cell proliferation index in cells of ovarian epithelial inclusions, ovarian surface epithelium, and tubal fimbriated epithelia, we examined a total of 20 ovarian cases containing 210 fallopian tube-derived ovarian epithelial inclusions, 48 mesothelium-derived ovarian surface epithelium samples, and 42 tubal fimbria epithelia by using MIB1 staining. Cell proliferation was significantly higher in fallopian tube-derived ovarian epithelial inclusions compared with mesothelium-derived ovarian surface epithelium samples but was significantly less than that in tubal fimbria epithelia. The cell proliferative index was 87.5-fold higher in fallopian tube-derived ovarian epithelial inclusions cells compared with mesothelium-derived ovarian surface epithelial cells (P<0.001). However, compared with tubal fimbria, the proliferative index in fallopian tube-derived ovarian epithelial inclusions showed an approximately threefold reduction (P<0.05; Table 3). As there were only a few cases of fallopian tube-derived ovarian surface epithelium, and proliferative indices of mesothelium-derived ovarian epithelial inclusions were similar to those of mesothelium-derived ovarian surface epithelium, these data were not included.
Secretory/Ciliated Cell Ratio in F-ovarian Epithelial Inclusions, Tubal Epithelia, and Ovarian Serous Tumors
Two methods were used to evaluate the secretory-to-ciliated cell ratios of fallopian tube-derived ovarian epithelial inclusions, tubal epithelia, and ovarian serous tumors: light microscopy by identifying ciliated cells directly, and tubulin immunohistochemical stain to identify ciliated cells. This evaluation was not applied to mesothelium-derived ovarian surface epithelium and ovarian epithelial inclusions, as these did not have either secretory or ciliated cells. Morphologically, the secretory-to-ciliated cell ratio was lowest in fallopian tube, and was significantly increased in fallopian tube-derived ovarian epithelial inclusions and serous cystadenomas, which had similar secretory-to-ciliated cell ratios. The secretory-to-ciliated cell ratio in serous borderline tumors (8.7±2.5) was only slightly higher, but all lesions showed significantly lower secretory-to-ciliated cell ratios than low-grade serous carcinomas (98±1.2). By tubulin immunohistochemical stain, a significant population of ciliated cells was present in fallopian tube-derived ovarian epithelial inclusions, cystadenomas, and borderline tumors with secretory-to-ciliated cell ratios of 5.5, 4.6, and 10.9, respectively (Table 4 and Figure 4). However, ciliated cells were basically all lost in low-grade serous carcinoma cases.
Discussion
‘Ovarian’ low-grade serous carcinomas are thought to evolve in a stepwise fashion, from ovarian epithelial inclusions or benign serous cystadenoma to serous borderline tumor, and eventually to carcinoma.11 This model is supported by the following facts: (1) similar mutations of KRAS and BRAF genes are present in serous borderline tumors and in adjacent serous cystadenoma epithelium;30 (2) compared with ovarian epithelial inclusions from ovaries with serous borderline tumors, ovarian epithelial inclusions from ovaries without non-neoplastic disease show significantly lower levels of epithelial cell aneusomy;31 and (3) the majority of low-grade serous carcinomas are associated with serous borderline tumors.32 The majority of serous cystadenomas are also thought to be derived from ovarian epithelial inclusions, as both display similar epithelial linings, and the diagnostic criteria dividing ovarian epithelial inclusions from serous cystadenoma are arbitrarily made at the 1 cm size threshold.33 The origin of ovarian epithelial inclusions can therefore provide insights into the origin of low-grade serous carcinoma. The coelomic metaplasia hypothesis has traditionally been used to explain the development of ovarian epithelial inclusions. In this hypothesis, the mesothelium overlying the ovary is assumed to invaginate into the underlying stroma to form ovarian epithelial inclusions. These cystic structures undergo metaplasia that results in the mesothelium being converted to Mullerian-type epithelium.33 These ovarian epithelial inclusions, with their newly acquired Mullerian phenotype, can then undergo malignant transformation. However, the aforementioned arguments against the ovarian surface epithelium as the origin of most ovarian epithelial cancers are similarly applicable to ovarian epithelial inclusions if it is assumed that ovarian epithelial inclusions are derived from the ovarian surface epithelium. In the present study, we evaluated the morphologic and immunophenotypic features of ovarian epithelial inclusions, ovarian surface epithelium, serous tumors, and distal tubal epithelium to gain a significant insight into the origin of low-grade serous carcinoma that are presently classified as ovarian origin by contemporary criteria. Based on the findings in this study, we discuss the following points.
First, we found that there are two types of ovarian epithelial inclusions and two types of ovarian surface epithelium, and both significantly differ in their proportional distribution. The first had mesothelial phenotype with an extremely low proliferative index: mesothelium-derived ovarian surface epithelium and mesothelium-derived ovarian epithelial inclusions. Mesothelium-derived ovarian surface epithelium was present in all ovarian sections studied. In contrast, mesothelium-derived ovarian epithelial inclusions were detected in only 22% of 856 ovarian epithelial inclusions studied. The second had tubal phenotype with a comparatively higher proliferative index: fallopian tube-derived ovarian surface epithelium and fallopian tube-derived ovarian epithelial inclusions. These cells were morphologically and immunophenotypically similar to tubal fimbria epithelial cells. The infrequent (4%) finding of ‘tubal-type’ epithelium in ovarian surface epithelium stands in stark to the high frequency (78%) of detecting this epithelial type in ovarian epithelial inclusions. These findings provide strong support for the concept that most of ovarian epithelial inclusions, and therefore lesions derived therefrom, are of tubal origin. The fact that the epithelial cells covering the ovarian surface can actually originate from the fallopian tube was an unexpected finding. Although this was only found in 2 (4%) of the 48 cases we studied, it does show that benign tubal epithelia are able to implant on the ovarian surface and architecturally simulate ‘ovarian surface epithelium’ microscopically. The small percentage of such fallopian tube-derived ovarian surface epithelium found in this study is similar to a recent study addressing the origin of high-grade serous carcinoma from patients with BRCA mutations.34 Additionally, we speculate that tubal epithelia implanted on the ovary are unstable and are easily sloughed off, as the ovaries are in a state of near-perpetual motion and agitation within the peritoneal cavity. In contrast, epithelia that are entrapped in the ovarian cortex are less amenable to easy physical detachment. This small proportion of fallopian tube-derived ovarian surface epithelium, and the notion that it is that population that has the most neoplastic potential, is supported by studies that have shown evidence of ‘oncogenic stress’ in only a small subset of ovarian epithelia interpreted as ‘ovarian surface epithelium’.29, 35
Second, ovarian epithelial inclusions are common in ovarian cortex including 22% of mesothelial and 78% tubal type. The question is now whether these fallopian tube-derived ovarian epithelial inclusions were derived from mesothelium-derived ovarian epithelial inclusions through a commonly believed metaplasia process. Although this possibility cannot be completely ruled out because of the descriptive nature of our study, we believe that the fallopian tube-derived ovarian epithelial inclusions are likely derived from tubal epithelia. The fact that we found more tubal-like epithelium in ovarian epithelial inclusions than in ovarian surface epithelium makes a strong argument that the fallopian tube-derived ovarian epithelial inclusions are not derived from the ovarian surface epithelium. The most straightforward explanation is that fallopian tube-derived ovarian epithelial inclusions represent intraovarian endosalpingiosis, which is well in line with the ideas expressed by Dubeau3 and Crum.10 Furthermore, if 78% fallopian tube-derived ovarian epithelial inclusions were truly originating from mesothelium-derived ovarian epithelial inclusions through a Mullerian metaplasia, the metaplastic process must be a common event and hybrid type of ovarian epithelial inclusions should be commonly found in the ovary. The fact that no hybrid or intermediate type of ovarian epithelial inclusions with both mesothelial and tubal phenotypes makes another strong argument that mesothelium-derived ovarian epithelial inclusions forming fallopian tube-derived ovarian epithelial inclusions through metaplasia is very unlikely. This is also supported by one previous observation that there were two types of ovarian epithelial inclusions, with one positive for calretinin and one negative for calretinin.36 In addition, mesothelium-derived ovarian epithelial inclusions seem not able to grow into a tumor mass as all these cystic structures have an extremely low cellular proliferative index and they are very unlikely to be the precursors of serous cystadenomas, borderline tumors, and low-grade serous carcinomas for the same reasons discussed above. In contrast, fallopian tube-derived ovarian epithelial inclusions showed comparable proliferative activity and immunophenotypes that were similar or identical to ‘ovarian’ serous tumors. From these perspectives, fallopian tube-derived ovarian epithelial inclusions are the more likely precursors to low-grade serous carcinoma.
Third, we found that ciliated cells, as are normally present in the fallopian tube, were also present in fallopian tube-derived ovarian surface epithelium, fallopian tube-derived ovarian epithelial inclusions, serous cysadenomas, and borderline tumors, with a significant increase in secretory-to-ciliated cell ratio from normal fallopian tube to fallopian tube-derived ovarian epithelial inclusions (P<0.001), but were very rare in low-grade serous carcinoma. The secretory-to-ciliated cell ratio was very similar between fallopian tube-derived ovarian epithelial inclusions and serous cystadenoma, and the secretory-to-ciliated cell ratios of both were only slightly lower than the secretory-to-ciliated cell ratio in serous borderline tumors. This was in contrast to low-grade serous carcinomas, whose epithelial component comprised almost entirely the secretory-type cells. High-grade serous carcinomas arising from the fallopian tube are thought to evolve via a clonal expansion of the secretory cell component of the tubal epithelium.14, 37, 38 Our findings suggest that low-grade serous carcinoma is similarly a clonal expansion of tubal-type secretory cells. The statistically significant increase in secretory-to-ciliated cell ratio that was observed between normal tubal epithelium and fallopian tube-derived ovarian epithelial inclusions suggests that a molecular event facilitating secretory cell expansion or ciliated cell suppression is present in fallopian tube-derived ovarian epithelial inclusions. The reduction in cilia with advancing tumor development might simply indicate an impaired maturation program. The similarity in the secretory-to-ciliated cell ratio between fallopian tube-derived ovarian epithelial inclusions and serous cystadenomas is consistent with the arbitrarity of the pathologic criteria (size threshold of 1 cm) by which these lesions are distinguished. Finally, the very high secretory-to-ciliated cell ratio in low-grade serous carcinoma, in conjunction with all of the aforementioned findings, is all consistent with the concept of a stepwise progression.
In summary, this study provides morphologic and immunophenotypic evidence that ovarian low-grade serous carcinoma is most likely originated from the tubal fimbria. In conjunction with evidence in the published literature, we propose a sequence of low-grade serous carcinoma development as follows: first, fallopian tubal epithelia, mostly from fimbriated end, implant on the ovarian surface. Two possibilities exist for how this detachment and implantation occurs: (1) given the close spatial relationship between the ovarian surface and the tubal fimbriated end, ovulation or non-ovulation-induced disruption of the ovarian surface may offer an opportunity for the adjacent tubal epithelium to detach and implant in the ovarian stroma;11 and (2) adhesion of tubal epithelium on the ovarian surface, from inflammation or other factors, and dynamic stromal growth around it may eventuate in fallopian tube-derived ovarian epithelial inclusion formation. The acquisition of KRAS or BRAF and possibly other mutations in fallopian tube-derived ovarian epithelial inclusions and serous cystadenomas result in their transformation to serous borderline tumors and ultimately, low-grade serous carcinomas.39, 40, 41, 42, 43 A small proportion of high-grade serous carcinomas may develop from low-grade serous carcinomas after the acquisition of additional mutations such as TP5311 and some high-grade serous carcinomas may arise in fallopian tube-derived ovarian epithelial inclusions when TP53 gene mutations occur in women with BRCA mutations.29 The secretory cell proliferations probably give rise to both low- and high-grade serous carcinomas and the degree of ciliated conversion is a function of the degree to which the genetic hits deregulate normal differentiation. Given that high-grade serous carcinomas are also increasingly accepted to be of tubal origin, our findings lend further credence to the concept that the cell of origin of most ovarian carcinomas is not a normal ovarian component. These findings may have significant implications for current ‘ovarian’ cancer-prevention strategies. Genetic and molecular studies are needed to further confirm the tubal origination of ovarian serous cancers.
References
Fathalla MF . Incessant ovulation--a factor in ovarian neoplasia? Lancet 1971;2:163–165.
Godwin AK, Testa JR, Handel LM, et al. Spontaneous transformation of rat ovarian surface epithelial cells: association with cytogenetic changes and implications of repeated ovulation in the etiology of ovarian cancer. J Natl Cancer Inst 1992;84:592–601.
Dubeau L . The cell of origin of ovarian epithelial tumours. Lancet Oncol 2008;9:1191–1197.
Carlson JW, Miron A, Jarboe EA, et al. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J Clin Oncol 2008;26:4160–4165.
Agoff SN, Mendelin JE, Grieco VS, et al. Unexpected gynecologic neoplasms in patients with proven or suspected BRCA-1 or -2 mutations: implications for gross examination, cytology, and clinical follow-up. Am J Surg Pathol 2002;26:171–178.
Callahan MJ, Crum CP, Medeiros F, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol 2007;25:3985–3990.
Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol 2007;31:161–169.
Finch A, Beiner M, Lubinski J, et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA 2006;296:185–192.
Rabban JT, Krasik E, Chen LM, et al. Multistep level sections to detect occult fallopian tube carcinoma in risk-reducing salpingo-oophorectomies from women with BRCA mutations: implications for defining an optimal specimen dissection protocol. Am J Surg Pathol 2009;33:1878–1885.
Crum CP . Intercepting pelvic cancer in the distal fallopian tube: theories and realities. Mol Oncol 2009;3:165–170.
Kurman RJ, Shih Ie M . The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 2010;34:433–443.
Kurman RJ, Shih Ie M . Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol 2008;27:151–160.
Folkins AK, Jarboe EA, Roh MH, et al. Precursors to pelvic serous carcinoma and their clinical implications. Gynecol Oncol 2009;113:391–396.
Jarboe EA, Folkins AK, Drapkin R, et al. Tubal and ovarian pathways to pelvic epithelial cancer: a pathological perspective. Histopathology 2008;53:127–138.
Lee Y, Miron A, Drapkin R, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol 2007;211:26–35.
Roh MH, Yassin Y, Miron A, et al. High-grade fimbrial-ovarian carcinomas are unified by altered p53, PTEN and PAX2 expression. Mod Pathol 2010;23:1316–1324.
Seidman JD, Russell P, Kurman RJ . Surface Epithelial Tumors of the Ovary, 5th edn Springer: New York, 2002.
Lang D, Powell SK, Plummer RS, et al. PAX genes: roles in development, pathophysiology, and cancer. Biochem Pharmacol 2007;73:1–14.
Bowen NJ, Logani S, Dickerson EB, et al. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol 2007;104:331–337.
Mittag J, Winterhager E, Bauer K, et al. Congenital hypothyroid female pax8-deficient mice are infertile despite thyroid hormone replacement therapy. Endocrinology 2007;148:719–725.
Nonaka D, Chiriboga L, Soslow RA . Expression of pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol 2008;32:1566–1571.
Tong GX, Devaraj K, Hamele-Bena D, et al. PAX8: a marker for carcinoma of Mullerian origin in serous effusions. Diagn Cytopathol 2010; e-pub ahead of print.
Laury AR, Hornick JL, Perets R, et al. PAX8 reliably distinguishes ovarian serous tumors from malignant mesothelioma. Am J Surg Pathol 2010;34:627–635.
Attanoos RL, Webb R, Dojcinov SD, et al. Value of mesothelial and epithelial antibodies in distinguishing diffuse peritoneal mesothelioma in females from serous papillary carcinoma of the ovary and peritoneum. Histopathology 2002;40:237–244.
Ordonez NG . Role of immunohistochemistry in distinguishing epithelial peritoneal mesotheliomas from peritoneal and ovarian serous carcinomas. Am J Surg Pathol 1998;22:1203–1214.
Ordonez NG . The diagnostic utility of immunohistochemistry and electron microscopy in distinguishing between peritoneal mesotheliomas and serous carcinomas: a comparative study. Mod Pathol 2006;19:34–48.
Levanon K, Ng V, Piao HY, et al. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene 2009;29:1103–1113.
van Agthoven T, Timmermans M, Foekens JA, et al. Differential expression of estrogen, progesterone, and epidermal growth factor receptors in normal, benign, and malignant human breast tissues using dual staining immunohistochemistry. Am J Pathol 1994;144:1238–1246.
Pothuri B, Leitao MM, Levine DA, et al. Genetic analysis of the early natural history of epithelial ovarian carcinoma. PLoS One 2010;5:e10358.
Ho CL, Kurman RJ, Dehari R, et al. Mutations of BRAF and KRAS precede the development of ovarian serous borderline tumors. Cancer Res 2004;64:6915–6918.
Korner M, Burckhardt E, Mazzucchelli L . Different proportions of aneusomic cells in ovarian inclusion cysts associated with serous borderline tumours and serous high-grade carcinomas support different pathogenetic pathways. J Pathol 2005;207:20–26.
Malpica A, Deavers MT, Lu K, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol 2004;28:496–504.
Scully RE . Pathology of ovarian cancer precursors. J Cell Biochem Suppl 1995;23:208–218.
Auersperg N . The origin of ovarian carcinomas: a unifying hypothesis. Int J Gynecol Pathol 2011.
Hutson R, Ramsdale J, Wells M . p53 protein expression in putative precursor lesions of epithelial ovarian cancer. Histopathology 1995;27:367–371.
Cao QJ, Jones JG, Li M . Expression of calretinin in human ovary, testis, and ovarian sex cord-stromal tumors. Int J Gynecol Pathol 2001;20:346–352.
Levanon K, Crum C, Drapkin R . New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol 2008;26:5284–5293.
Chen EY, Mehra K, Mehrad M, et al. Secretory cell outgrowth, PAX2 and serous carcinogenesis in the Fallopian tube. J Pathol 2010;222:110–116.
Singer G, Oldt III R, Cohen Y, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst 2003;95:484–486.
Alvarez AA, Moore WF, Robboy SJ, et al. K-ras mutations in Mullerian inclusion cysts associated with serous borderline tumors of the ovary. Gynecol Oncol 2001;80:201–206.
Haas CJ, Diebold J, Hirschmann A, et al. In serous ovarian neoplasms the frequency of Ki-ras mutations correlates with their malignant potential. Virchows Arch 1999;434:117–120.
Mayr D, Hirschmann A, Lohrs U, et al. KRAS and BRAF mutations in ovarian tumors: a comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol 2006;103:883–887.
Sieben NL, Macropoulos P, Roemen GM, et al. In ovarian neoplasms, BRAF, but not KRAS, mutations are restricted to low-grade serous tumours. J Pathol 2004;202:336–340.
Acknowledgements
Dr Jie Li is a PhD candidate co-trained at University of Arizona, USA and Shandong University, China. The project was supported in part by Better Than Ever Fund, P30 CA23074 from Arizona Cancer Center and Department of Pathology, University of Arizona Startup fund to WZ, and Shandong University, China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, J., Abushahin, N., Pang, S. et al. Tubal origin of ‘ovarian’ low-grade serous carcinoma. Mod Pathol 24, 1488–1499 (2011). https://doi.org/10.1038/modpathol.2011.106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2011.106
Keywords
This article is cited by
-
A dog oviduct-on-a-chip model of serous tubal intraepithelial carcinoma
Scientific Reports (2020)
-
PTEN loss in the fallopian tube induces hyperplasia and ovarian tumor formation
Oncogene (2018)
-
BRCA1 expression, proliferative and apoptotic activities in ovarian epithelial inclusions
Journal of Ovarian Research (2017)
-
Gankyrin facilitates follicle-stimulating hormone-driven ovarian cancer cell proliferation through the PI3K/AKT/HIF-1α/cyclin D1 pathway
Oncogene (2016)
-
Endosalpingiosis (A Rare Pathology that Mimic Others): Could it be a Precursor of Cancer?
Indian Journal of Gynecologic Oncology (2016)