Abstract
Regenerating gene family members 1 (REG Iα) and 4 (REG IV) are overexpressed in a subset of gastric cancers. However, comparative characterization of the expression of these family proteins has remained unclear. Therefore, we aimed to elucidate not only the association between REG protein expression and mucin phenotype but also their significance as a prognostic marker for patients with gastric cancer. The expression of REG Iα, REG IV, CDX2, MUC2, and MUC5AC in gastric cancer tissues was examined by immunohistochemistry. The relationship between REG protein expression and clinicopathological parameters or mucin phenotype was then analyzed. REG Iα and REG IV expression was positive in 33 (52%) and 31 (49%) of 63 gastric cancers examined, respectively. REG Iα expression was significantly related to venous invasion and tumor stage, whereas REG IV expression showed no relationship to clinicopathological features. With regard to mucin phenotype, REG IV expression was significantly correlated with MUC2 and CDX2 expression, suggesting an association with the intestinal mucin phenotype of gastric cancer. On the other hand, REG Iα expression had no correlation with MUC2, CDX2, or MUC5AC in gastric cancer tissues. Expression of REG Iα but not REG IV was an independent predictor of poor outcome in patients with gastric cancer. In addition, patients with gastric cancer negative for both REG Iα and REG IV expression had a significantly better outcome than patients positive for either REG Iα or REG IV. Profiling of REG protein expression is useful to for prognostication of patients with gastric cancer.
Similar content being viewed by others
Main
The regenerating gene (Reg) was originally isolated from regenerating rat pancreatic islets,1 and its gene product was shown to have a trophic effect on not only islet but also gastric epithelial cells.2, 3 Recently, many Reg-related genes have been isolated and shown to constitute a multigene family.4 Among human REG family proteins, REG Iα and REG IV are reportedly overexpressed in a subset of gastric cancer.5, 6, 7, 8, 9 Moreover, several microarray analyses have recently isolated REG Iα and REG IV as novel genes that are overexpressed in gastric cancer tissues,10, 11 suggesting that both genes have important functions in gastric carcinogenesis. Indeed, we have previously clarified that REG Iα acts as a trophic and/or anti-apoptotic factor in the development of gastric cancer,12 and others have reported that REG IV protein confers cell resistance to chemotherapeutic agents,9 suggesting that these proteins are both associated with tumor progression. It is noteworthy that in non-neoplastic tissues REG Iα and REG IV are commonly expressed not only in endocrine cells but also in metaplastic cells that frequently accompany gastric cancer lesions,5, 8, 12, 13 implying a possible link between REG protein expression and mucin phenotype of gastric lesions. In addition, there is increasing speculation as to whether REG Iα and REG IV protein expression may be applicable as a prognostic marker for patients with various malignancies.6, 7, 9, 14, 15 However, comparative characterization of the expression of these proteins in gastric cancer tissues has not yet been carried out. Therefore, in this study, we attempted to clarify not only the association between REG protein expression and mucin phenotype but also their prognostic significance in patients with gastric cancer.
Materials and methods
Patients, Tissue Samples, and Histology
Sixty-three patients with gastric cancer (42 men, 21 women; mean age 65.3, range 36–83 years) who underwent surgery at Dokkyo University School of Medicine between 1997 and 2001 were enrolled. Patients with other malignant diseases were excluded, as were patients who had received preoperative treatment such as chemotherapy and radiation therapy. This study was carried out with the approval of the Dokkyo University Surgical Pathology Committee, and informed consent was obtained from all patients.
The resected specimens were fixed in 10% neutral buffered formalin and embedded in paraffin. Multiple hematoxylin-eosin-stained sections of all 63 lesions were examined. The tissue sections including the invasive front of tumor were subjected to immunostaining. The following factors were determined for all patients and lesions: age, sex, tumor location, Lauren's histological classification, tumor invasion, lymph node metastases, and patient survival. The tumor stage was according to the International Union Against Cancer TNM staging system.16 The clinicopathological features of the patients were summarized in Table 1.
Immunohistochemistry
Immunohistochemical staining for REG Iα, REG IV, CDX2, MUC2, and MUC5AC was performed with a LSAB-2 kit (Dako, Marseille, France) as described previously.5 In brief, 4-μm-thick sections were placed on slides, deparaffinized, and dehydrated. They were then placed in 0.01 mol/l citrate buffer (pH 6.0) and treated by microwave heating (MI-77; Azumaya, Tokyo, Japan; 400 W, 95°C) for 10 and 40 min to facilitate antigen retrieval for REG Iα and Ki-67, respectively, whereas they received no treatment with microwave heating for the immunostaining of ssDNA. Then, the sections were followed by pretreatment with 0.3% H2O2 in methanol for 20 min at room temperature to quench endogenous peroxidase activity. The sections were incubated with 1% bovine serum albumin in phosphate-buffered saline (PBS) for 30 min, and then with anti-REG Iα (dilution 1:1000), anti-REG IV (R&D Systems, Minneapolis, MN, USA; dilution 1:50), anti-CDX2 (BioGenex, San Ramon, CA, USA; dilution 1:20), anti-MUC2 (Novocastra, Newcastle, UK; dilution 1:100), and anti-MUC5AC (Novocastra; dilution 1:100) for 1 h. Thereafter, the sections were incubated with biotinylated secondary antibody for 15 min, washed with PBS, and treated with peroxidase-conjugated streptavidin for 20 min. Finally, the sections were incubated in 3,3′-diaminobenzidine tetrahydrochloride with 0.05% H2O2 for 3 min and then counterstained with Mayer's hematoxylin. The monoclonal antibody for human REG Iα protein was generated as previously reported, against human REG Iα protein corresponding to positions 23–166 of the deduced human REG Iα protein.17 The specificity of the antibody was proven not only by western blot analysis17 but also by immunohistochemistry.18
The percentage of cancer cells stained with each antibody was evaluated. A result was considered positive if at least 10% of cells were stained.8 When fewer than 10% of cancer cells were stained, immunostaining was considered negative. For mucin-phenotype analysis, gastric cancers were classified into four groups as follows: G-type, positive for MUC5AC alone; I-type, positive for MUC2 alone; GI-type, positive for both MUC5AC and MUC2; N-type, negative for both MUC5AC and MUC2.
Statistical Analysis
Statview 5.0J statistical software (Abacus Concepts Inc., Berkeley, CA, USA) was used for all analyses. χ2-Analyses were performed to investigate the relationship between REG proteins expression and clinicopathological features or immunohistochemical data. All values were expressed as the mean±s.e.m., and the significance of differences between two groups was assessed using Mann–Whitney U-test. Overall survival rate was calculated using the Kaplan–Meier method and analyzed by the log-rank test. Univariate and multivariate Cox regression analyses were used to examine whether REG protein expression was an independent predictor of overall survival. The significant predictors identified in univariate analysis were then subjected to multivariate analysis. The level of statistical significance was set at P<0.05.
Results
Relationship Between REG Protein Expression and Clinicopathological Features in Patients with Gastric Cancer
Immunoreactivity for REG Iα and REG IV proteins was detected in various types of gastric cancer (Figure 1). REG Iα and REG IV proteins were both detected in goblet-like vesicles or in the perinuclear region of gastric cancer cells.
Immunostaining of REG Iα (a, c, and e) and REG IV (b, d, and f) proteins in gastric cancer tissues. (a and b) Well-differentiated adenocarcinoma. Immunoreactivity of REG Iα (a) and REG IV (b) is localized in goblet-like vesicles (arrows) or perinuclear (arrowheads) in the cytoplasm of tumor cells. (c and d) Poorly differentiated adenocarcinoma. The cytoplasm of tumor cells is positive for REG Iα (c) and REG IV (d). (e and f) Serial sections of signet-ring cell carcinoma. REG Iα (e) and REG IV (f) are coexpressed in the signet-ring cells. Bars=100 μm.
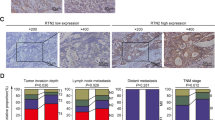
Of the 63 gastric cancer tissue samples examined, 33 (52%) and 31 (49%) were positive for REG Iα and REG IV proteins, respectively. As shown in Figure 1e and f, some gastric cancers were positive for both REG Iα and REG IV proteins. Indeed, expression of REG Iα tended to correlate with that of REG IV in the gastric cancer tissues examined (Table 2). With regard to clinicopathological features, REG Iα expression was significantly associated with the prevalence of venous invasion and high tumor stage, whereas REG IV expression showed no relationship to any of the clinicopathological features investigated (Table 3).
Relationship Between REG Protein Expression and Mucin Phenotype in Gastric Cancer
We investigated the association between REG protein expression and mucin phenotype using gastric and intestinal mucin markers. Gastric and intestinal markers were detected in 44 of 63 (70%) cases for MUC5AC, 32 (51%) cases for MUC2, and 44 (70%) cases for CDX2. Positivity for REG IV protein was significantly correlated with positivity for MUC2 and CDX2 in gastric cancer tissues (Figure 2, Table 4). However, positivity for REG Iα protein showed no correlation with MUC2, CDX2, or MUC5AC, although it showed a tendency to be correlated with CDX2 expression.
On the basis of the expression of MUC5AC and MUC2, we classified the 63 gastric cancers phenotypically as G-type (22; 35%), I-type (10; 16%), GI-type (22; 35%), and N-type (9; 14%). REG IV-positive gastric cancer was significantly of the GI-type (18 of 31; 58%, P<0.001). In addition, REG IV-positive gastric cancer tented to be of the I-type (7 of 31; 23%, P=0.151). However, REG Iα-positive cancer showed no specific mucin phenotype (Table 5).
Prognostic Value of REG Protein Expression in Patients with Gastric Cancer
To evaluate the prognostic significance of REG protein expression in patients with gastric cancer, we constructed Kaplan–Meier curves (Figure 3). We found that patients with REG Iα-positive gastric cancer had a significantly worse outcome than those without (Figure 3a). In addition, the patients with REG IV-positive gastric cancer tended to show a poor outcome, but not to a significant degree (Figure 3b).
We next examined whether REG Iα expression was an independent factor predictive of overall survival in patients with gastric cancer. Univariate analysis indicated that venous invasion, lymph node metastasis, tumor stage, and REG Iα expression were significantly predictive of overall survival. Moreover, multivariate analysis revealed that tumor stage and REG Iα expression were independently predictive of overall survival (Table 6).
Finally, we investigated whether combination of REG Iα and REG IV expression was of value for prognostication of patients with gastric cancer. Interestingly, patients who were negative for both REG Iα and REG IV showed a significantly better outcome than patients in the other groups (Figure 4). Moreover, double negative expression of REG Iα and REG IV was independently predictive of overall survival in patients with gastric cancer when subjected to multivariate Cox regression, including factors such as venous invasion, lymph node metastasis, and tumor stage (P=0.0076, hazard ratio=8.403, 95% confidence interval 1.761–40.000).
Overall survival according to combination of REG Iα (a) and REG IV (b) expression in patients with gastric cancer. Patients with gastric cancer negative for both REG Iα and REG IV expression showed a significantly better outcome than other patients positive for at least REG Iα or REG IV (a, P=0.043; b, P=0.005 by log-rank test).
Discussion
REG family proteins commonly possess three disulfide bonds among six conserved cysteine residues and form a Drickamer motif that is common in the calcium-dependent lectin (C-type lectin) superfamily.19 At present, REG family proteins are classified into four subfamilies according to their primary structures,20 and their overexpression has been reported in neoplastic or inflamed tissues in various organs including the stomach, colorectum, bile duct, and pancreas.5, 6, 7, 8, 14, 21, 22 Interestingly, microarray analyses suggest that expression of the REG Iα and REG IV genes is prominently upregulated during the development of gastric cancer,10, 11 and in fact we found immunohistochemically that not only REG Iα but also REG IV protein was overexpressed in a considerable number of gastric cancers. However, we also found that REG Iα and REG IV were not always coexpressed in a subset of gastric cancers. Thus, although REG Iα and REG IV proteins belong to the same family, their expression profiles apparently differ in gastric cancer tissues.
In this study, we focused on the association between REG protein expression and the mucin phenotype of the gastric cancer because REG proteins are commonly expressed in the goblet or metaplastic cells that produce abundant mucin in the gastrointestinal tract.8, 12 Compatible with a previous report,8 REG IV expression was significantly associated with the intestinal mucin phenotype of gastric cancer. However, we found no association between REG Iα expression and gastrointestinal mucin-phenotype markers, MUC2 or MUC5AC, in gastric cancers. Although we cannot explain this discrepancy, the regulatory mechanisms of REG Iα and REG IV expression may differ. With regard to REG Iα expression in gastric cancer cells, STAT3 signaling is suggested to be crucial in vitro;23 on the other hand, EGFR signaling is closely associated with REG IV expression not only in gastric but also in colorectal cancer cells.9, 24, 25 Moreover, immunohistochemical analyses revealed that REG Iα and REG IV expression is indeed associated with phosphorylated STAT3 and EGFR expression in gastric cancer tissues, respectively.9, 23 Although the clinicopathological significance of REG protein expression is not fully understood, it may be interesting for practical use to consider REG Iα and REG IV as biomarkers of intracellular signaling rather than of the mucin phenotype.
Accumulating evidence suggests that REG Iα and REG IV commonly have functions in tumor progression by exerting trophic and/or anti-apoptotic effects on gastric cancer cells.12, 24, 25 Accordingly, another important question is whether REG Iα and REG IV proteins are reliable markers for prognostication of patients with gastric cancer. We found that REG Iα but not REG IV is an independent prognostic marker for patients with gastric cancer. On the other hand, we also found that patients with REG IV-positive gastric cancer tended to show a poor outcome, although not to a statistically significant degree. Therefore, we also investigated whether a combination of REG Iα and REG IV expression would be beneficial for more precise prediction of outcome in patients with gastric cancer. In comparison with REG Iα alone, combination of REG Iα and REG IV did not dramatically improve the predictive ability. However, combination of the two REG proteins was clearly advantageous for distinguishing between good and poor outcome in the REG Iα-negative group. Interestingly, 9Mitani et al. have reported that REG IV expression does not predict patient survival, but significantly predicts resistance to chemotherapeutic agents. In this regard, we favor the combination of REG Iα and REG IV rather than REG Iα alone for strict delineation of patients with gastric cancer who are likely to have a poor outcome.
In summary, our present data show that REG Iα expression, having no association with gastrointestinal mucin phenotype, is a significantly useful prognostic marker in patients with gastric cancer when compared with REG IV expression in association with the intestinal mucin phenotype in such patients. On the other hand, it is noteworthy that REG IV expression appears to be a good marker for predicting susceptibility to chemotherapy in patients with gastric cancer,9 although this issue with regard to REG Iα expression still remains to be elucidated. Therefore, in the further studies we would like to investigate whether the profiling of REG protein expression is useful not only for prognostication but also for providing individualized treatment in patients with gastric cancer.
References
Terazono K, Yamamoto H, Takasawa S, et al. A novel gene activated in regenerating islets. J Biol Chem 1988;263:2111–2114.
Watanabe T, Yonemura Y, Yonekura H, et al. Pancreatic beta-cell replication and amelioration of surgical diabetes by Reg protein. Proc Natl Acad Sci USA 1994;91:3589–3592.
Fukui H, Kinoshita Y, Maekawa T, et al. Regenerating gene protein may mediate gastric mucosal proliferation induced by hypergastrinemia in rats. Gastroenterology 1998;115:1483–1493.
Kinoshita Y, Ishihara S, Kadowaki Y, et al. Reg protein is a unique growth factor of gastric mucosal cells. J Gastroenterol 2004;39:507–513.
Fukui H, Fujii S, Takeda J, et al. Expression of Reg Iα protein in human gastric cancers. Digestion 2004;69:177–184.
Dhar DK, Udagawa J, Ishihara S, et al. Expression of regenerating gene I in gastric adenocarcinomas: correlation with tumor differentiation status and patient survival. Cancer 2004;100:1130–1136.
Yonemura Y, Sakurai S, Yamamoto H, et al. REG gene expression is associated with the infiltrating growth of gastric carcinoma. Cancer 2003;98:1394–1400.
Oue N, Mitani Y, Aung PP, et al. Expression and localization of Reg IV in human neoplastic and non-neoplastic tissues: Reg IV expression is associated with intestinal and neuroendocrine differentiation in gastric adenocarcinoma. J Pathol 2005;207:185–198.
Mitani Y, Oue N, Matsumura S, et al. Reg IV is a serum biomarker for gastric cancer patients and predicts response to 5-fluorouracil-based chemotherapy. Oncogene 2007;26:4383–4393.
Takaishi S, Wang TC . Gene expression profiling in a mouse model of Helicobacter-induced gastric cancer. Cancer Sci 2007;98:284–293.
Oue N, Hamai Y, Mitani Y, et al. Gene expression profile of gastric carcinoma: identification of genes and tags potentially involved in invasion, metastasis, and carcinogenesis by serial analysis of gene expression. Cancer Res 2004;64:2397–2405.
Sekikawa A, Fukui H, Fujii S, et al. REG Iα protein may function as a trophic and/or anti-apoptotic factor in the development of gastric cancer. Gastroenterology 2005;128:642–653.
Kämäräinen M, Heiskala K, Knuutila S, et al. RELP, a novel human REG-like protein with up-regulated expression in inflammatory and metaplastic gastrointestinal mucosa. Am J Pathol 2003;163:11–20.
Oue N, Kuniyasu H, Noguchi T, et al. Serum concentration of Reg IV in patients with colorectal cancer: overexpression and high serum levels of Reg IV are associated with liver metastasis. Oncology 2007;72:371–380.
Minamiya Y, Kawai H, Saito H, et al. REG1A expression is an independent factor predictive of poor prognosis in patients with non-small cell lung cancer. Lung Cancer 2008;60:98–104.
Sobin LH, Wittekind CH, (eds) TNM Classification of Malignant Tumors (5th edn) Wiley-Liss: New York, 1997;59–62.
Watanabe T, Yonekura H, Terazono K, et al. Complete nucleotide sequence of human reg gene and its expression in normal and tumoral tissues. J Biol Chem 1990;265:7432–7439.
Satomura Y, Sawabu N, Ohta H, et al. The immunohistochemical evaluation of PSP/reg-protein in normal and diseased human pancreatic tissues. Int J Pancreatol 1993;13:59–67.
Drickamer K . Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem 1988;263:9557–9560.
Unno M, Yonekura H, Nakagawara K, et al. Structure, chromosomal localization, and expression of mouse reg genes, reg I and reg II. A novel type of reg gene, reg II, exists in the mouse genome. J Biol Chem 1993;268:15974–15982.
Kimura N, Yonekura H, Okamoto H, et al. Expression of human regenerating gene mRNA and its product in normal and neoplastic human pancreas. Cancer 1992;70:1857–1863.
Harada K, Zen Y, Kanemori Y, et al. Human REG I gene is up-regulated in intrahepatic cholangiocarcinoma and its precursor lesions. Hepatology 2001;33:1036–1042.
Sekikawa A, Fukui H, Fujii S, et al. REG Iα protein mediates an anti-apoptotic effect of STAT3 signaling in gastric cancer cells. Carcinogenesis 2008;29:76–83.
Nanakin A, Fukui H, Fujii S, et al. Expression of the REG IV gene in ulcerative colitis. Lab Invest 2007;87:304–314.
Bishnupuri KS, Luo Q, Murmu N, et al. Reg IV activates the epidermal growth factor receptor/Akt/AP-1 signaling pathway in colon adenocarcinomas. Gastroenterology 2006;130:137–149.
Acknowledgements
We thank Chiaki Matsuyama, Ayako Shimizu, Takako Ono, Midori Katayama, Nozomi Nagashima, Atsuko Kikuchi (Department of Surgical and Molecular Pathology, Dokkyo University School of Medicine, Tochigi, Japan), and Sachiko Miyahara and Shin-ichi Yasuda (Institute of International Education and Research, Dokkyo University School of Medicine, Tochigi, Japan) for their excellent technical and secretarial assistance. This work was supported in part by grants-in-aid for Scientific Research 19790494 and 20590747 from the Ministry of Education, Culture, Sports, Science and Technology, Japan. We are grateful to Dr Okamoto in Tohoku University Graduate School of Medicine, Sendai, Japan, for providing anti-REG Iα antibody.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/conflict of interest
The authors state no conflicts of interest in this study.
Rights and permissions
About this article
Cite this article
Yamagishi, H., Fukui, H., Sekikawa, A. et al. Expression profile of REG family proteins REG Iα and REG IV in advanced gastric cancer: comparison with mucin phenotype and prognostic markers. Mod Pathol 22, 906–913 (2009). https://doi.org/10.1038/modpathol.2009.41
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2009.41
Keywords
This article is cited by
-
Prognostic role of regenerating gene-I in patients with stage-IV head and neck squamous cell carcinoma
Diagnostic Pathology (2016)
-
Gene expression analysis of a Helicobacter pylori-infected and high-salt diet-treated mouse gastric tumor model: identification of CD177 as a novel prognostic factor in patients with gastric cancer
BMC Gastroenterology (2013)
-
Validation of four candidate pancreatic cancer serological biomarkers that improve the performance of CA19.9
BMC Cancer (2013)
-
REG Iα is a biomarker for predicting response to chemotherapy with S-1 plus cisplatin in patients with unresectable stage IV gastric cancer
British Journal of Cancer (2013)
-
Enhanced RegIV Expression Predicts the Intrinsic 5-Fluorouracil (5-FU) Resistance in Advanced Gastric Cancer
Digestive Diseases and Sciences (2013)