Abstract
As a definite immunoprofile of this tumor is missing, the histopathologic diagnosis of intrahepatic cholangiocarcinoma is difficult. The aim of this study was to explore E- and N-cadherin expressions in intrahepatic bile duct tumors, and to determine their potential interest in differential diagnosis. Normal liver tissue, 5 cirrhosis with ductular reaction, 5 focal nodular hyperplasia, 5 bile duct hamartomas, 5 bile duct adenomas, and 45 intrahepatic cholangiocarcinomas from Causasian patients were studied. Tissue-microarrays including 20 esophageal, 86 gastric, 8 small bowel, 64 colonic, 18 pancreatic, 6 gallbladder, and 7 extrahepatic biliary tract adenocarcinomas, 22 hepatocellular carcinomas, and normal tissues were constructed. Immunohistochemistry was performed using E-cadherin, N-cadherin, NCAM, Hep Par1, and cytokeratins 7, 19 and 20. Immunoblot analysis of frozen liver tissues was performed to control the specificity of E- and N-cadherin antibodies used. In normal liver, epithelial cells of intrahepatic bile ducts, whatever their caliber, as well as hepatocytes, coexpressed E- and N-cadherins at their plasma membranes. In cirrhosis, ductular reactions completely expressed E- and N-cadherins. All the benign lesions and 30 of the 45 intrahepatic cholangiocarcinomas (23/29 peripheral and 7/16 hilar) also expressed N-cadherin. E-cadherin was detected in all the lesions. The expression of N-cadherin at the plasma membrane of tumor cells was significantly more frequent in peripheral than in hilar intrahepatic cholangiocarcinomas (P=0.003). Among noncholangiocarcinomas, only 1% gastric and 66% gallbladder adenocarcinomas and all the hepatocellular carcinomas expressed N-cadherin at the membrane of tumor cells. Finally, for the diagnosis of intrahepatic cholangiocarcinomas, the specificity value of membranous expression of N-cadherin was 88%, whereas that of the combination cytokeratin 7/membranous N-cadherin was 98%. In the gastrointestinal and liver tract, membranous N-cadherin is restricted to the hepatocytes and intrahepatic biliary cells. In combination with cytokeratin 7 and Hep Par1, N-cadherin is a reliable tool for the histopathological diagnosis of primary hepatic tumors.
Similar content being viewed by others
Main
Intrahepatic cholangiocarcinoma is a highly malignant adenocarcinoma arising from the bile duct epithelial cells of the intrahepatic biliary system, may be through a hyperplasia—dysplasia—carcinoma sequence.1 Intrahepatic cholangiocarcinomas can be clinically classified into two types according to their localization in the liver: (1) hilar cholangiocarcinomas that originate from the liver hilum or in close vicinity of the bifurcation of the right and left hepatic ducts and (2) peripheral cholangiocarcinomas that arise from the second or more distal branches of the biliary tree.2 However, hilar cholangiocarcinomas (Klatskin tumors) are categorized as extrahepatic cholangiocarcinomas by the International Classification of Diseases for Oncology.3 The immunohistochemical profile as well as the molecular mechanisms underlying the tumor progression of intrahepatic cholangiocarcinomas are still poorly delineated. A better knowledge of their diagnostic markers could facilitate an earlier identification of cholangiocarcinomas, help predict the prognosis and make decisions on adjuvant therapies for those patients. In this context, a definite profile of intrahepatic cholangiocarcinomas is still missing. Today, their immunohistochemical profile resides in the coexpression of cytokeratins 7, 19 and 20;4, 5 however, this profile remains unsatisfactory as it is shared by a variety of liver metastases of digestive tumors.
Cadherins form a superfamily of specialized membrane glycoproteins mediating calcium-dependent cell–cell adhesion and expressed in tissue specific manner.6 Among the various cadherins likely to be expressed by epithelial cells in human, both E- and N-cadherins are constantly present at the membrane of hepatocytes and tumor cells of hepatocellular carcinomas.7, 8, 9, 10, 11, 12 Interestingly, apart from E-cadherin, little is known of cadherins expressed by the biliary tree.8, 13 In the face of studies showing the tissue specificity of cadherin subtypes, we were prompted to extend the study of E- and N-cadherin expression by the normal epithelial cells of the different segments of biliary tree as well as by tumors. To this end we compared the expression of E- and N-cadherins (1) by the intrahepatic branches to that of extrahepatic bile ducts and (2) by the intrahepatic cholangiocarcinomas to that of other gastrointestinal tract tumors using tissue micro-array technology and immunoblotting. We conclude that N-cadherin is a relevant tool in diagnostic practice to distinguish primary intrahepatic biliary tumors from all other gastrointestinal tract tumors.
Patients and methods
Patients
A total of 5 patients with bile duct adenoma, 5 patients with focal nodular hyperplasia, 5 patients with bile duct hamartomas (von Meyenburg complex) and 45 patients with intrahepatic cholangiocarcinomas including 16 patients with hilar cholangiocarcinomas having undergone a surgical resection at the Centre Hospitalier Universitaire de Nantes and Hôpital Beaujon, Clichy, France, were studied. Three extrahepatic metastases of intrahepatic cholangiocarcinomas from these patients were also studied. The 45 patients with intrahepatic cholangiocarcinomas (17 women and 28 men; mean age=65 years; range=36–80 years) were diagnosed on clinical, computerized tomodensitometry and histological criteria. In parallel studies, normal liver tissue and extrahepatic bile ducts of six patients having undergone partial liver resection for metastasis of colorectal carcinoma or pancreatic surgery and six liver cirrhosis were studied as controls. In addition, resection specimens from primary adenocarcinomas and metastases of adenocarcinomas from various sites of the digestive tract from 231 patients were used to build tissue microarrays. Finally, needle liver biopsies from 24 patients with metastatic lung or breast adenocarcinoma were included in the study. Samples of cholangiocarcinomas from two patients were snap frozen in liquid nitrogen, for determining expression of E- and N-cadherins by immunoblot analysis. This study was performed in accordance with local ethical guidelines. Snap-frozen tissues were collected by the BioBank Network of Institut Régional du Cancer Nantes-Atlantique, according to the guidelines of the French Ethics Committee for Research on human tissue.14 Clinical information was obtained from medical records. Tumor stage was determined according to tumor-node-metastasis stage and the postoperative survival time was assessed. Information about nodal involvement was available for 27 of the tumors, of which 15 were node positive and 12 were node negative.
Gross Features of Cholangiocarcinomas
Cholangiocarcinomas were grossly classified into three categories according to the Japanese study group of primary liver cancer.15 The mass-forming type (peripheral: n=28; hilar: n=7) is an expansile and solid nodule or mass in the hepatic parenchyma. The tumor borders between the cancerous and the noncancerous portions are relatively clear. The periductal-infiltrating type (hilar: n=8), which exhibits diffuse infiltration along the portal pedicle, is usually associated with biliary stricture. In the intraductal-growth type (peripheral: n=1; hilar n=1), the tumors are confined within the dilated part of an intrahepatic large bile duct, with no or mild tumorous extension beyond the bile duct walls. This tumor is also known as intraductal papillary cholangiocarcinoma. The affected bile ducts usually show marked localized dilatation.
Tissue Microarray Construction
Paraffin-embedded tissue blocks from the 45 patients undergoing surgery were used for this study. Four cholangiocarcinoma tissue microarrays were constructed. Briefly, the construction of each tissue microarray was performed as follows: paraffin blocks containing areas consisting of pure invasive carcinoma were identified on corresponding hematoxylin-eosin-stained sections. Areas of interest of the tumor, rich in nonnecrotic glands, were identified and marked on the source block. The source block was cored, and a 1-mm core was transferred to the recipient master block using the Beecher Microarrayer (Beecher Instruments, Silver Springs, MD, USA). Three cores of tumor were arrayed per specimen. In addition, a core of normal tissue was also systematically sampled.
Control tissue microarrays included one esophageal adenocarcinoma tissue microarray (n=20), five gastric adenocarcinoma tissue microarrays (n=86) including liver metastasis (n=4), one small bowel carcinoma tissue microarray (n=8), one extrahepatic biliary tract (n=7) and gallbladder (n=6) tissue microarray, one pancreatic ductal adenocarcinoma tissue microarray (n=18), one hepatocellular carcinoma (n=22) tissue microarray and four colon adenocarcinoma (n=64) tissue microarrays including liver metastasis (n=4).
Immunohistochemistry
Immunohistochemistry was performed on 5-μm paraffin sections of each tissue microarray described above. Slides were stained with the following primary antibodies: cytokeratin 7 (clone OV-TL 12/30, dilution 1:100; Dako, Glostrup, Denmark), cytokeratin 19 (clone BA17, dilution 1:50; Dako), cytokeratin 20 (clone K20.8, dilution 1:25; Dako), Hep Par 1 (clone OCH1G5, dilution 1:50; Dako), NCAM (clone 123C3, dilution 1:100; Monosan, Uden, The Netherlands), E-cadherin (clone NCH-38, dilution 1:100; Dako) and N-cadherin (clone 3B9, dilution 1:50; Zymed, San Francisco, CA, USA). The immunological reactions were visualized with the Envision detection system (Dako) and 3,3-diaminobenzidine tetrahydrochloride as the chromogen. The sections were counterstained with Mayer's hematoxylin. As negative control, omission of primary antibody was performed.
Immunohistochemistry Scoring
For markers expressed at the cell membrane or within the cytoplasm, the scoring method of Sinicrope et al16 was applied for evaluating both the intensity of immunohistochemical staining and proportion of stained epithelial cells. The staining intensity was subclassified as 1 (weak), 2 (moderate) and 3 (strong). Positive cells were quantified as a percentage of the total number of epithelial cells, and assigned to one of four categories: 0, 0–10; 1, 10–33; 2, 33–75; 3, >75%. The percentage of positivity of the tumor cells and staining intensity were multiplied to generate the immunoreactive score for each tumor specimen. Finally, we verified that the results of immunohistochemistry performed on tissue microarrays paralleled those of the corresponding full sections, based on the random selection of 10 cases. For a diagnostic purpose, a cut-off of 10% stained tumor cells was chosen to define a positive tumor.
Immunoblot Analysis
For total protein extraction, samples taken from two patients with intrahepatic cholangiocarcinoma and one sample of normal liver were lyzed in RIPA buffer supplemented with protease inhibitors and centrifuged as previously described.17 Proteins (50 μg) were separated by electrophoresis on 10% polyacrylamide gels and transferred onto nitrocellulose membranes (0.45 μm porosity; Bio-Rad, Hercules, CA, USA) using Trans-blot apparatus (Bio-Rad). After blocking, membranes were incubated with primary antibodies against E-cadherin endodomain (clone 4A2C7, dilution 1:1000; Zymed) and N-cadherin endodomain (clone 3B9, dilution 1:250; Zymed), and then with a horseradish-peroxidase conjugated, goat anti-mouse antibody (dilution 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The immunoreactive proteins were detected on films using an enhanced chemiluminescence substrate according to the manufacturer's instructions (Roche Diagnostics, Meylan, France).
Statistics
The diagnostic value of each pertinent immunoprofile was analyzed according to its sensitivity (true positive cases/true positive cases+false-negative cases), specificity (true negative cases/true negative cases+false-positive cases) and positive predictive value (true positive cases/true positive cases+false-positive cases). Comparisons between biomarker expression patterns and clinicopathologic parameters were evaluated using χ2-test. A P-value of less than 0.05 was considered as statistically significant.
Results
Immunohistochemical Pattern of Normal Intrahepatic Bile Ducts and Cholangiocarcinomas
Normal bile ducts
In the normal liver tissue, both hepatocytes and normal intrahepatic bile ducts, whatever their caliber, were strongly immunoreactive for E- and N-cadherins, localized at the basolateral domain of the plasma membrane (Figure 1a and b). They also expressed both cytokeratins 7 and 19, but did not express cytokeratin 20. Normal bile ducts did not express either NCAM or Hep Par 1.
Normal extrahepatic bile ducts were strongly immunoreactive for E-cadherin, localized at the basolateral domain of the plasma membrane but they did not express N-cadherin (Figure 1c and d). They also expressed cytokeratins 7 and 19, and few epithelial cells also expressed cytokeratin 20.
Ductular reaction
Ductular reaction describes the expanded population of epithelial cells at the interface of the biliary tree and the hepatocytes in diseased states including cirrhosis. It has a distinct tubular and almost glandular-like structure with poorly defined lumen and no basement membrane.18 Ductular reactions consisting of a subset of intermediate hepatobiliary cells (Figure 2a), including stem cells, were localized in cirrhotic liver using NCAM immunohistochemistry.19, 20 All cells of ductular reactions scored positive for both N- (Figure 2b) and E-cadherins at their plasma membrane.
Benign liver diseases
Five focal nodular hyperplasias, five bile ducts adenomas and five bile duct hamartomas (von Meyenburg complex) were included in this study. In focal nodular hyperplasia, hepatocytes and ductular reaction at the margins of fibrous scars were completely positive for both N- and E-cadherins. The intermediate hepatobiliary cells of ductular reaction also expressed NCAM. In bile duct adenomas, as well as in bile duct hamartomas (Figure 3a), the epithelial cells strongly expressed N- and E-cadherins at the basolateral membrane. All epithelial cells also expressed cytokeratins 7 and 19. There was no expression of cytokeratin 20 or NCAM.
Intrahepatic cholangiocarcinomas
N- (Figure 3b–d) and E-cadherins were expressed at the plasma membrane of tumor cells in 30 (66%) and 45 (100%) intrahepatic cholangiocarcinomas, respectively. The level of expression of each marker was variable. No cholangiocarcinoma showed cytoplasmic expression for N-cadherin. When comparing the distribution of these markers between peripheral and hilar cholangiocarcinomas, the expression of N-cadherin was significantly more frequent in peripheral cholangiocarcinomas (23 of 29 (79%)) than in hilar cholangiocarcinomas (7 of 16 (43%); χ2, P=0.003). N-cadherin was expressed by 25 of the 35 mass-forming cholangiocarcinomas, 3 of the 8 periductal infiltrating type and the 2 intraductal growth type. There was no statistical difference in the expression of N-cadherin according to the gross pattern of the tumors. N-cadherin was focally expressed in 18 intrahepatic cholangiocarcinomas (10–33% positive cells: n=9; 33–75% positive cells: n=7; >75% positive cells: n=2) and diffusely expressed in 11 cases. Among the 10 cases studied in full sections, 5 expressed focally N-cadherin. In these five cases, focality of the staining was also documented by examination of cores included in tissue microarrays. N-cadherin was focally expressed in the seven positive hilar cholangiocarcinomas (10–33% positive cells: n=2; 33–75% positive cells: n=5).
The 45 intrahepatic cholangiocarcinomas (100%) were immunoreactive for cytokeratins 7 and 19 whereas only 7 (15%) expressed cytokeratin 20. A few isolated tumor cells expressed NCAM in 12 intrahepatic cholangiocarcinomas (26%). Three intrahepatic cholangiocarcinomas (6%) focally expressed Hep Par1.
The unexpected immunohistochemical profile of intrahepatic cholangiocarcinomas, ie, the expression of N-cadherin at the plasma membrane associated with cytokeratins 7 and 19 within the cytoplasm of tumor cells, prompted us to extend the immunostaining for N-cadherin to other primary digestive tumors and their liver metastases using tissue microarray technology. The results, summarized in Table 1, showed that one primary gastric adenocarcinoma (1%), four gallbladder adenocarcinomas (66%) and all hepatocellular carcinomas (100%) expressed N-cadherin at the plasma membrane of tumor cells. None of the colonic adenocarcinomas, esophageal adenocarcinomas, pancreatic ductal adenocarcinomas, extrahepatic biliary adenocarcinomas, liver metastases of gastric and colonic tumors expressed N-cadherin at the membrane of tumor cells. Finally, 3 pancreatic ductal adenocarcinomas (17%) and 15 gastric adenocarcinomas (17%) showed a faint cytoplasmic expression of N-cadherin.
In addition, the expression of N-cadherin was assessed in liver biopsies from 12 patients with intrahepatic metastases of TTF-1-positive lung adenocarcinoma and 12 patients with intrahepatic metastases of breast adenocarcinoma expressing progesterone or estrogen receptors. All these metastases were N-cadherin negative (Figure 4).
Lack of N-cadherin expression at the plasma membrane of tumor cells of an intrahepatic metastasis lung adenocarcinoma (a) which was TTF1 positive (b). Only hepatobiliary cells of ductular reaction expressed N-cadherin at their membrane (arrows). A perivascular nerve fiber was also N-cadherin positive (arrowhead).
Diagnostic value of the membranous N-cadherin and cytoplasmic cytokeratin 7 immunostaining
As all intrahepatic cholangiocarcinomas expressed both cytokeratins 7 and 19, we compared the diagnostic value of combining membranous N-cadherin and cytoplasmic cytokeratin 7 immunostaining, to that of N-cadherin immunostaining alone. The sensitivity, specificity and predictive positive value of the combination of membranous N-cadherin and cytoplasmic cytokeratin 7 expression for intrahepatic cholangiocarcinomas were 67, 98 and 86%, respectively. The sensitivity, specificity and predictive positive value of the membranous expression of N-cadherin were 67, 88 and 53%, respectively.
Identification of E-Cadherin and N-Cadherin by Immunoblot Analysis
Immunoblot analysis using antibodies specific for the endodomain of E- and N-cadherins (Figure 5) showed (1) the full-length of E- and N-cadherins (120 and 136 kDa, respectively) and (2) the remaining endodomain of E- and N-cadherins after ectodomain shedding (33 and 35 kDa, respectively).
Immunoblot analysis of E-cadherin (a) and N-cadherin (b) in tumor tissue from a patient with intrahepatic cholangiocarcinoma. Equal amount of protein was loaded in each lane as determined by protein analysis and immunoblot for actin (data not shown). Numbers on the left indicate the molecular weight markers.
Relationship Between the Expression of Cadherins and the Clinicopathologic Parameters
The expression of each biomarker was semiquantitatively evaluated according to the scale described in Patients and methods. The expression level of each biomarker in normal intrahepatic bile duct systematically scored in all tissue microarrays served as a normal reference for the evaluation of a biomarker expression in intrahepatic cholangiocarcinomas. In the normal tissue, the labeling index of E- and N-cadherins were 9 and 6, respectively. The clinicopathologic parameters evaluated were gross pattern, histological grade of intrahepatic cholangiocarcinomas, tumor stage, node metastasis and visceral metastasis.
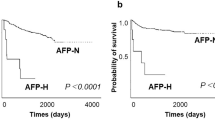
When compared to normal tissue, the expression level of membranous E- and N-cadherins was downregulated in 21 (46%) and 36 (80%) of primary intrahepatic cholangiocarcinomas, respectively. No correlation was found between any clinicopathologic parameter including the histological grade and either E- or N-cadherin expression. Two of the three metastases of intrahepatic cholangiocarcinomas showed a downregulation of a specific cadherin, ie, E-cadherin in one case and N-cadherin in the other.
Of the 45 patients with intrahepatic cholangiocarcinomas, 25 were available for survival studies. Mean survival times was 28 months (range 1–92 months). The death of most patients was due to metastasis in 14 cases and to the disabling side effects of the liver disease in 11 cases. No correlation was noted between loss of E- or N-cadherin expression, and metastases occurrence or behavior.
Discussion
The important findings of this study are as follows: (1) the normal intrahepatic bile ducts as well as the tumors derived thereof express both E- and N-cadherins whereas extrahepatic bile ducts express only E-cadherin; (2) the expression of N-cadherin appears early in the differentiation process of intrahepatic biliary epithelial cells and (3) N-cadherin is a relevant tool to distinguish primary intrahepatic biliary tumors from other epithelial tumors of the digestive tract.
For the first time, we show a membranous expression of N-cadherin in association with that of E-cadherin by intrahepatic biliary epithelial cells. In fact, a few epithelia express N-cadherin, such as lens cells21 and tubular renal cells.22 In addition, in our study, immunoblot analysis confirms that the monoclonal antibody used, ie, clone 3B9 from Zymed Laboratories is specific for N-cadherin. In the normal liver, it detects two bands of 136 and 35 kDa corresponding to the full length of N-cadherin and its proteolytically cleaved cytoplasmic domain, respectively.23, 24 Interestingly, Kozyraki et al8 using a different monoclonal antibody which detects a unique band with an apparent molecular weight of 129 kDa were able to observe the N-cadherin expression in hepatocytes, but not in intrahepatic biliary epithelial cells. Taken together, our findings show that in the gastrointestinal tract, N-cadherin expression is liver specific because both hepatocytes and intrahepatic biliary epithelial cells strongly express this marker at their plasma membrane. An interesting point is our finding that N-cadherin is not expressed by extrahepatic bile ducts. This can be explained by the different embryological origins of extrahepatic and intrahepatic bile ducts.25 In this context, it must be emphasized that in addition to N-cadherin, some other plasma membrane proteins of biliary epithelial cells present a heterogeneous pattern of expression along the biliary tract. For example, Scoazec et al,26 showed expression of α5 integrin chain in the intrahepatic biliary epithelial cells in contrast to their extrahepatic counterparts. In line with a gradient of N-cadherin expression along the normal biliary tract, we found that peripheral cholangiocarcinomas were mostly membranous N-cadherin positive whereas hilar cholangiocarcinomas were equally membranous N-cadherin positive or negative and extrahepatic cholangiocarcinomas were membranous N-cadherin negative.
Cirrhosis is a valuable model to examine hepatocytic and bile duct lineages as the so-called ductular reaction. Ductular reaction is thought to be an important source of progenitor cells that can repopulate both the bile duct and hepatocytic lineages.20, 27 This model describes that NCAM-positive stem cells, an analog of the oval cells of rodent models, differentiate both as NCAM-positive, cytokeratin 19-positive bile duct lineage and NCAM-positive, Hep Par 1-positive hepatocytic lineage.20 Ductular reaction is easily detected through its NCAM immunostaining.18 Interestingly, in our study, all cells of ductular reaction expressed both E- and N-cadherins, suggesting that N-cadherin appears early in the differentiation of liver cells and participates in the migration of precursors of hepatocytes and ductular biliary epithelial cells.
As the differential diagnosis of primary intrahepatic cholangiocarcinomas from liver metastases of digestive tract adenocarcinomas is difficult by using only histological features, several studies have put forward some immunohistochemical markers such as various cytokeratins or mucins as a diagnostic aid.4, 5, 28, 29 However, no marker is sufficiently specific in this respect. In contrast the membranous expression of N-cadherin by tumor cells provides a promising tool for the diagnosis of intrahepatic cholangiocarcinomas. Indeed, our tissue microarrays study including adenocarcinomas from various sites of the digestive tract showed that neither primary tumors nor their liver metastases expressed N-cadherin at the plasma membrane of tumor cells. However, in our study, we obtained a higher differential diagnostic specificity using a combination of cytokeratin 7 and membranous N-cadherin to distinguish intrahepatic cholangiocarcinomas and hilar cholangiocarcinomas from most hepatocellular carcinomas and most digestive tract adenocarcinomas including pancreatic adenocarcinomas which are one of the most difficult differential diagnostic problems. In apparent contrast with our study, an abnormal N-cadherin expression was recently reported in some pancreatic and extrahepatic biliary tract adenocarcinomas.30 However, this expression was localized within the cytoplasm of the tumor cells, sparing their plasma membrane. This abnormal pattern of N-cadherin expression was considered as an evidence for an epithelial to mesenchymal transition in cancer cells. In this context, the assessment of a membranous localization is of utmost importance to differentiate intrahepatic cholangiocarcinomas from metastases of digestive tumors. Among all the gastrointestinal tract tumors assessed in our study, only one gastric adenocarcinoma expressed N-cadherin at the membrane of tumor cells. Interestingly, it has been previously reported that N-cadherin is expressed by α-foetoprotein-positive gastric hepatoid adenocarcinomas.31
Another diagnostic issue is to discriminate primary intrahepatic cholangiocarcinomas from hepatocellular carcinomas. As the hepatocytes also express N-cadherin at their plasma membrane, this marker cannot ‘per se’ distinguish hepatocellular carcinomas from intrahepatic cholangiocarcinomas. Moreover, cytokeratin 7 is detected in some hepatocellular carcinomas, potentially deriving from hepatic progenitor cells.32 Hepatocyte paraffin-1 (Hep Par 1) is a monoclonal antibody that reacts with a hepatocyte specific epitope,33 recently identified as the urea cycle enzyme carbamoyl phosphate synthetase 1.34 This marker is known to be largely confined to hepatocellular carcinoma.35, 36 Thus in this setting, Hep Par 1 is needed, in combination with N-cadherin and cytokeratin 7, to assess histologically problematic primary liver tumors, ie, hepatocellular carcinomas exhibiting a pseudoglandular or poorly differentiated morphology.
In conclusion, the expression of N-cadherin at the plasma membrane of tumor cells strongly argues for the primary origin of a liver tumor. A panel of antibodies including N-cadherin, cytokeratin 7 and Hep Par 1 can solve most diagnostic problems among cholangiocarcinomas, hepatocellular carcinomas and metastases of digestive tumors.
References
Zen Y, Adsay NV, Bardadin K, et al. Biliary intraepithelial neoplasia: an international interobserver agreement study and proposal for diagnostic criteria. Mod Pathol 2007;20:701–709.
Okuda K, Kubo Y, Okazaki N, et al. Clinical aspects of intrahepatic bile duct carcinoma including hilar carcinoma: a study of 57 autopsy-proven cases. Cancer 1977;39:232–246.
Welzel TM, McGlynn KA, Hsing AW, et al. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst 2006;98:873–875.
Rullier A, LeBail B, Fawaz R, et al. Cytokeratin 7 and 20 expression in cholangiocarcinomas varies along the biliary tract but still differs from that in colorectal carcinoma metastasis. Am J Surg Pathol 2000;24:870–876.
Lau SK, Prakash S, Geller SA, et al. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum Pathol 2002;33:1175–1181.
Takeichi M . Cadherins: cell adhesion receptors as a morphogenetic regulator. Science 1991;251:1451–1455.
Yamaoka K, Nouchi T, Tazawa J, et al. Expression of gap junction protein connexin 32 and E-cadherin in human hepatocellular carcinoma. J Hepatol 1995;22:536–539.
Kozyraki R, Scoazec JY, Flejou JF, et al. Expression of cadherins and alpha-catenin in primary epithelial tumors of the liver. Gastroenterology 1996;110:1137–1149.
Ihara A, Koizumi H, Hashizume R, et al. Expression of epithelial cadherin and alpha- and beta-catenins in nontumoral livers and hepatocellular carcinomas. Hepatology 1996;2:1441–1447.
Endo K, Ueda T, Ueyama J, et al. Immunoreactive E-cadherin, alpha-catenin, beta-catenin, and gamma-catenin proteins in hepatocellular carcinoma: relationships with tumor grade, clinicopathologic parameters, and patients' survival. Hum Pathol 2000;31:558–565.
Matsumura T, Makino R, Mitamura K . Frequent down-regulation of E-cadherin by genetic and epigenetic changes in the malignant progression of hepatocellular carcinomas. Clin Cancer Res 2001;7:594–599.
Wei Y, Van Nhieu JT, Prigent S, et al. Altered expression of E-cadherin in hepatocellular carcinoma: correlations with genetic alterations, beta-catenin expression, and clinical features. Hepatology 2002;36:692–701.
Ashida K, Terada T, Kitamura Y, et al. Expression of E-cadherin, α-catenin, β-catenin, and CD44 (standard and variant isoforms) in human cholangiocarcinomas: an immunohistochemical study. Hepatology 1998;27:974–982.
ANAES. Recommandations pour la cryoconservation de cellules et tissus tumoraux dans le but de réaliser des analyses moléculaires. Ann Pathol 2001;21:184–201.
Liver Cancer Study Group. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 3rd edn. Kanehara Publications: Tokyo, 1994.
Sinicrope FA, Ruan SB, Cleary KR, et al. Bcl2 and p53 oncoprotein expression during colorectal tumorogenesis. Cancer Res 1995;55:237–241.
Blanchot-Jossic F, Jarry A, Masson D, et al. Up-regulated expression of ADAM17 in human colon carcinomas: co-expression with EGFR in neoplastic and endothelial cells. J Pathol 2005;207:156–163.
Roskams TA, Theise ND, Balabaud C, et al. Nomenclature of the finest branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology 2004;39:1739–1745.
Roskams TA, van den Oord JJ, De Vos R, et al. Neuroendocrine features of reactive bile ductules in cholestatic liver disease. Am J Pathol 1990;137:1019–1025.
Zhou H, Rogler LE, Teperman L, et al. Identification of hepatocytic and bile ductular cell lineages and candidate stem cells in bipolar ductular reactions in cirrhotic human liver. Hepatology 2007;45:716–724.
Volk T, Geiger B . A 135 kDa membrane protein of intercellular adherens junctions. EMBO J 1984;3:2249–2260.
Biddlestone L, Fleming S . Morphological evidence that ACAM is a major intercellular adhesion molecule in human kidney. J Pathol 1991;164:9–15.
Uemura K, Kihara T, Kuzuya A, et al. Characterization of sequential N-cadherin cleavage by ADAM10 and PS1. Neurosci Lett 2006;402:278–283.
Reiss K, Maretzky T, Ludwig A, et al. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and β-catenin nuclear signalling. EMBO J 2005;24:742–752.
Desmet V . Embryology of the liver and intrahepatic biliary tract, and an overview of malformations of the bile duct. In: MacIntyre N, Benhamou JP, Bircher J, Rizzeto M, Rodes J (eds). Oxford Textbook of Clinical Hepatology, Vol. 1 Oxford University Press: Oxford, 1991, pp 497–501.
Scoazec JY, Bringuier AF, Medina JF, et al. The plasma membrane polarity of human biliary epithelial cells: in situ immunohistochemical analysis and functional implications. J Hepatol 1997;26:543–553.
Haruna Y, Saito K, Spaulding S, et al. Identification of bipotential progenitor cells in human liver development. Hepatology 1996;23:476–481.
Shibahara H, Tamada S, Higashi M, et al. MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma—mass forming type. Hepatology 2004;39:220–229.
Fucich LF, Cheles MK, Thung SN, et al. Primary vs metastatic hepatic carcinoma. An immunohistochemical study of 34 cases. Arch Pathol Lab Med 1994;118:927–930.
Nakajima S, Doi R, Toyoda E, et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin Cancer Res 2004;10:4125–4133.
Yanagimoto K, Sato Y, Shimoyama Y, et al. Co-expression of N-cadherin and alpha-fetoprotein in stomach cancer. Pathol Int 2001;51:612–618.
Durnez A, Verslype C, Nevens F, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology 2006;49:138–151.
Wennerberg AE, Nalesnik MA, Coleman WB . Hepatocyte paraffin 1: a monoclonal antibody that reacts with hepatocytes and can be used for differential diagnosis of hepatic tumors. Am J Pathol 1993;143:1050–1054.
Butler SL, Dong H, Cardona D, et al. The antigen for Hep Par 1 antibody is the urea cycle enzyme carbamoyl phosphate synthetase 1. Lab Invest 2008;88:78–88.
Leong ASY, Sormunen RT, Tsui WMS, et al. Hep Par 1 and selected antibodies in the immunohistological distinction of hepatocellular from cholangiocarcinoma, combined tumours and metastatic carcinoma. Histopathology 1998;33:318–324.
Chu PG, Ishizawa S, Wu E, et al. Hepatocyte antigen as a marker of hepatocellular carcinoma: an immunohistochemical comparison to carcinoembryonic antigen, CD10, and alpha-fetoprotein. Am J Surg Pathol 2002;26:978–988.
Acknowledgements
This study was supported in part by a grant from a Projet Hospitalier de Recherche Clinique (BRD 04/6/P) and la Ligue Départementale Contre le Cancer de la Loire Atlantique.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mosnier, JF., Kandel, C., Cazals-Hatem, D. et al. N-cadherin serves as diagnostic biomarker in intrahepatic and perihilar cholangiocarcinomas. Mod Pathol 22, 182–190 (2009). https://doi.org/10.1038/modpathol.2008.123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2008.123
Keywords
This article is cited by
-
The crosstalk between cholangiocytes and hepatic stellate cells promotes the progression of epithelial-mesenchymal transition and periductal fibrosis during Clonorchis sinensis infection
Parasites & Vectors (2024)
-
Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA)
Nature Reviews Gastroenterology & Hepatology (2016)