Abstract
The next generation of needle-free mucosal vaccines is being rationally designed according to rules that govern the way in which the epitopes are recognized by and stimulate the genital mucosal immune system. We hypothesized that synthetic peptide epitopes extended with an agonist of Toll-like receptor 2 (TLR-2), that are abundantly expressed by dendritic and epithelial cells of the vaginal mucosa, would lead to induction of protective immunity against genital herpes. To test this hypothesis, we intravaginally (IVAG) immunized wild-type B6, TLR-2 (TLR2−/−) or myeloid differentiation factor 88 deficient (MyD88−/−) mice with a herpes simplex virus type 2 (HSV-2) CD8+ T-cell peptide epitope extended by a palmitic acid moiety (a TLR-2 agonist). IVAG delivery of the lipopeptide generated HSV-2-specific memory CD8+ cytotoxic T cells both locally in the genital tract draining lymph nodes and systemically in the spleen. Moreover, lipopeptide-immunized TLR2−/− and MyD88−/− mice developed significantly less HSV-specific CD8+ T-cell response, earlier death, faster disease progression, and higher vaginal HSV-2 titers compared to lipopeptide-immunized wild-type B6 mice. IVAG immunization with self-adjuvanting lipid-tailed peptides appears to be a novel mucosal vaccine approach, which has attractive practical and immunological features.
Similar content being viewed by others
Introduction
Herpes simplex virus type 2 (HSV-2) remains the most common sexually transmitted pathogen that initiates infection at genital mucosal surfaces. Genital herpes is the most common sexually transmitted disease worldwide.1 In the absence of strong local immunity, recurrent ulcerative lesions, produced by viral reactivation from dorsal root ganglia, predispose and increase the risk of acquiring human immunodeficiency virus (HIV)2, 3, 4 and papillomavirus associated with cervical carcinoma.5, 6 In some cases, HSV-2 infections are fatal to newborns and cause encephalitis or meningitis in adults.7 Despite the availability of many intervention strategies, such as behavioral education, condom usage, and standard antiviral drug therapies, the transmission rate of herpes has continued to rise during the past three decades.1, 8 Thus, there remains a serious need to develop an alternative immunoprophylactic or immunotherapeutic vaccine strategy to control the spread of herpes.2, 3, 8 However, there is currently no vaccine available against genital herpes.
Over the last five decades, numerous conventional candidate live attenuated and killed herpes vaccines that were efficacious in animal models failed in clinical trials (reviewed in references4, 5). The majority of these vaccines is administered parenterally and can induce strong systemic immune responses. However, they do not generate significant immunity at the mucosal site of infection nor in the local draining lymph nodes of the genital tract (GT), that many experts see as necessary to prevent the transmission or limit the severity of sexually transmitted diseases.6, 9, 10, 11, 12, 13, 14, 15 We hypothesize that an efficient subunit intravaginal (IVAG) vaccine would induce local immunity at—or at least close to—the site of genital infection thus maximizing its ability to protect the GT from subsequent HSV-2 challenge. However, the progress toward an IVAG vaccine still faces significant challenges including: (i) the overall low immunogenicity of subunit formulations delivered into the GT compared to other mucosal routes (e.g., intranasal route);6, 16, 17, 18 (ii) the imperative requirement for a safe and effective mucosal adjuvant;4, 19, 20 and (iii) a better understanding of key effector immune molecules of the vaginal mucosal immune system.21, 22, 23
The initial host response to vaccination occurs after Toll-like receptors (TLRs) on dendritic cells (DCs) are stimulated through specific TLR agonists. In the last decade, there has been an interest in targeting TLR in the GT to induce protective immunity against sexually transmitted diseases, including HSV-2 (reviewed in references6, 23). Thus, recent studies have investigated the TLR expression patterns in the GT and reported that both DC and epithelial cells of the vaginal and cervical mucosa abundantly express bioactive TLR-2.23, 24, 25, 26, 27, 28 In the meantime, we and others have established that parenteral delivery of self-adjuvanting peptides extended by a TLR-2 agonist (palmitic acid moiety), can induce significant protective immunity (reviewed in reference4). Moreover, intranasal administration of palmitoyl-tailed peptide epitopes induced strong local and systemic T-cell responses.4, 29, 30, 31, 32 We have also found that, in vitro, antibody blocking of TLR-2, but not TLR-4, abrogates DC presentation of lipopeptide epitopes to T cells.33 We therefore hypothesized that IVAG lipopeptide vaccines targeting TLR-2 would induce local and systemic T-cell immunity and protect the female GT against herpes.
As a model antigen, we used a prototype CD4+ T-helper CD8+ T-cytotoxic chimeric epitope lipopeptide that consists of the HSV glycoprotein B (gB) CD8+ cytotoxic T cell (CTL) immunodominant epitope (gB498–505) in line with the Pan DR peptide (PADRE), a universal CD4+ helper T cell (Th) epitope. This prototype lipopeptide molecule was linked in turn to three palmitic acid moieties and designated helper-cytotoxic-T-lymphocyte chimeric epitopes (Th-CTL lipopeptide).34 We show here that IVAG delivery of Th-CTL lipopeptide elicited both local and systemic HSV-specific effector and memory CD8+ T-cell responses, reduced virus replication in the GT, and subsequently protected from overt signs of genital disease. Induction of interferon (IFN)-γ-producing CD8+ T cells by Th-CTL lipopeptide was dependent on TLR2 and myeloid differentiation factor 88 (MyD88). Our results highlight the potential of self-adjuvanting lipopeptides as a novel, noninvasive needle-free IVAG vaccine approach to prevent the transmission and/or limit the severity of sexually transmitted diseases.
Results
IVAG immunization with peptide epitopes extended by a palmitic acid moiety induced HSV-specific CD8+ T cells in the lymph nodes that drain the genital tract
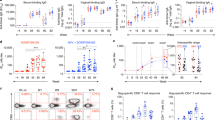
B6 mice were immunized IVAG two times at 21 days interval with equimolar amount of Th-CTL lipopeptide, Th-CTL nonlipidated peptide control, Th peptide (PADRE), or CTL peptide alone (n=10 per group). Ten days after the second immunization, inguinal lymph nodes were harvested and analyzed for gB498–505 peptide- and HSV-specific T-cell responses by IFN-γ-ELISpot assay. The group immunized with Th-CTL lipopeptide developed significantly more gB498–505- and HSV-specific IFN-γ-producing CD8+ T cells in the inguinal lymph nodes than any other group (P≤0.05 for all; Figure 1a) The Th-CTL nonlipidated peptide group had more gB498–505 peptide-specific CD8+ T cells than the other control groups (P<0.05) but these CD8+ T cells did not respond to HSV-2. There were no significant differences between the other control groups. In addition, a mixture of Th-CTL nonlipidated peptide analog+palmitic acid did not induce CD8+ T-cell responses (not shown), thus underlying the requirement for a covalent linkage between the TLR-2 agonist and the peptide backbone.
Glycoprotein B (gB)498–505- and herpes simplex virus (HSV)-2-specific CD8+ T cells induced in the draining lymph nodes following intravaginal lipopeptide immunization. B6 mice (n=10) were immunized twice with helper-cytotoxic-T-lymphocyte chimeric epitopes (Th-CTL lipopeptide; LIPO), Th-CTL peptide (PEP), CTL peptide alone or Th peptide alone with an interval of 2 weeks between immunizations. Ten days after the second immunization, inguinal lymph nodes were harvested and gB498–505- and HSV-specific interferon (IFN)-γ-producing CD8+ T cells and their CTL activity were measured. (a) Shows the IFN-γ producing CD8+ T cells as measured by enzyme-linked immunosorbent spot (ELISpot) assay and; (b) shows the CTL activity specific to either gB498–505 peptide (top) or the vaccinia virus expressing gB (VVgB) (bottom) as measured by a 51Cr release assay using EL-4 as target cells at effector–target ratios (E:T) of 3, 10, 30, and 90 respectively. Bars represent mean values for each group (± s.e.m.). Asterisk (*) indicates P<0.05.
Next, B6/H2b (EL4) target cells were either pulsed with gB498–505 peptide or infected with a vaccinia virus-expressing gB (VVgB) and tested in a standard 51Cr release assay for recognition by CD8+ T cells from the lymph nodes of IVAG-immunized mice (Figure 1b). The CD8+ CTL lines generated by the lipopeptide lysed both gB498–505 peptide-pulsed and VVgB-infected target cells, confirming the immunogenicity of the lipopeptide-administered IVAG and the functional presentation of endogenously expressed gB498–505 epitope to T cells.
Induction of CD8+ cytotoxic T cells that recognize endogenously processed HSV-gB at distant systemic sites
To determine if the T-cell responses induced by IVAG immunization with Th-CTL lipopeptide were disseminated to distant systemic sites and whether they will recognize endogenously processed gB, spleen (SPL) were harvested 10 days following the second immunization and analyzed for VVgB-specific CD8+ CTL by 51Cr assay, as above. Significantly, more VVgB-specific CD8+ CTL activity was detected in the SPL of the Th-CTL lipopeptide-immunized group than in the group immunized with nonlipidated peptide analog (P=0.003; Figure 2a). Additionally, no VVgB-specific CTL activity was detected in the SPL of mice immunized with Th peptide or CTL peptide alone.
Induction of herpes simplex virus (HSV)-specific CD8+ TC1 cells in the spleen following intravaginal immunization with helper-cytotoxic-T-lymphocyte chimeric epitopes (Th-CTL lipopeptide). B6 mice (n=5) were immunized as described in Figure 1. Ten days after the second immunization spleens were harvested and in (a) vaccinia virus expressing glycoprotein B (VVgB)-specific CD8+ CTL was measured in spleen by 51Cr release assay at an effector–target (E:T) ratio of 30 in (b) frequency of HSV-gB498–505-specific CD8+ T cells were measured by tetramer. CD8+ T cells were stimulated in vitro with heat-inactivated virus pulsed Ag-presenting cells (APCs) and stained with a PE-labeled anti-CD8 mAb followed by an FITC-labeled HSV-gB498–505/H2-Kb tetramer. Cells were analyzed using a FACSCalibur with a total of 4 × 105 events collected for each point. Histograms of the percentage of CD8+/Tetramer+ cells representative of two independent experiments is shown. Bars represent mean values for each group (± s.e.m.). Asterisk (*) indicates significant difference between groups (P≤0.05). (c) Represents the cytokines profile produced by CD8+ T cells. Spleen derived CD8+ T cells were stimulated in vitro with the target gB498–505 peptide-loaded H2b-irradiated splenocytes for 5 days at 37°C in 5% CO2. The amounts of interleukin (IL)-2, IL-4, IL-12, and interferon (IFN)-γ secreted into the culture medium were measured in a specific sandwich ELISA according to the manufacturer's instructions. The data are the representative of two independent experiments. The P values compare the amounts of each cytokine between Th-CTL lipopeptide PEP, CTL, and Th peptide-immunized mice.
We next performed an objective enumeration of HSV-gB498–505-specific CD8+ T cells induced by Th-CTL lipopeptide, Th-CTL peptide, Th, or CTL peptides alone, using the MHC tetramer-staining assay. After the second IVAG immunization, SPL cells from each group were restimulated in vitro with heat-inactivated HSV-2 and stained with HSV-gB498–505 H2-Kb tetramer followed by CD8 staining. As shown in Figure 2b, approximately 8% of total CD8+ T cells detected in SPL of Th-CTL lipopeptide-immunized mice were HSV-gB498–505 H2-Kb tetramer positive. The other groups had a low percentage of CD8+ HSV-gB498–505 H2-Kb tetramer positive cells. As expected, staining for the irrelevant OVA257–264/H2-Kb tetramer was at a nonsignificant level (0.12–0.043%, not shown).
We assessed whether Th-CTL lipopeptide IVAG immunization would induce a polarized memory type 1 (Tc1) or type 2 (Tc2) cytotoxic T-cell responses. Ten days after the second IVAG immunization with Th-CTL lipopeptide, local draining inguinal lymph node (DLN)-derived CD8+ T cells were isolated from each group of mice and restimulated in vitro with HSV-1 gB498–505 peptide-pulsed DCs. The amounts of IFN-γ, interleukin (IL)-2, IL-4, and IL-12 released in the T-cell culture medium were determined in a sandwich ELISA assay. IVAG administration of Th-CTL lipopeptide induced high amounts of IFN-γ, IL-2, and IL-12 but only a small amount of IL-4 (Figure 2c). The corresponding nonlipidated peptide failed to induce any significant cytokine response. The levels of IFN-γ, IL-2, and IL-12 correlated with CTL activity shown in Figure 2a. Collectively, these data show that IVAG immunization with Th-CTL lipopeptide not only elicited cytolytic CD8+ T cells (Figure 2a) but also induced a pattern of cytokines (Figure 2c) consistent with a polarized Tc1 response.
Functional characterization of in vivo HSV-specific CD8+ T cells
Two additional approaches were used to compare the effector functions of CD8+ T cells after IVAG immunization. First, HSV-specific CTL responses were compared in SPL using an in vivo CTL assay known as a CFSE CTL assay. This assay measures lysis of specific HSV-infected target cells adoptively transferred into immunized animals that have effector CD8+ T cells. The assay was performed 30 days after the second immunization. IVAG immunization with Th-CTL lipopeptide resulted in approximately 48% lysis of specific targets compared to approximately 7–8.5% lysis in the controls when measured 1 h postadoptive transfer of HSV-infected targets (Figure 3a). Thus, the CTL response of Th-CTL immunized animals was approximately sixfold higher than in control. As the in vivo CTL assay does not require in vitro expansion, this approach is considered direct evidence that IVAG Th-CTL lipopeptide immunization induced systemic CD8+ T-cell responses to HSV.
Functional characterization of herpes simplex virus (HSV)-specific CD8+ T cells induced following intravaginally (IVAG) immunization of self-adjuvanting lipopeptide. (a) Syngeneic spleen cells were infected with HSV-2 and labeled with CFSE (2.5 μm). To control for antigen specificity, noninfected syngeneic spleen cells were labeled with a lower concentration of CFSE (0.25 μm). A 1:1 mixture of each target cell population was injected i.v. into helper-cytotoxic-T-lymphocyte chimeric epitopes (Th-CTL lipopeptide) immunized and control groups of B6 mice 30 d post-IVAG immunization. One hour after injection, spleen cells were harvested from individual mice and acquired on a FACS. The percentage of specific lysis was determined as mentioned in Methods. The number shown in each plot is the mean of percent antigen specific lysis, determined from gate M1, and that was observed in four mice per group. (b and c) Single cell suspensions of spleen were analyzed for expression of early and late marker of activation; CD44 and CD69 (dashed lines), or isotype control (dark line). Following FACS analysis, the forward angle and side scatter gates were set on the CD3+ population. Gating on the CD8+ population determined the proportion of CD8+ T cells that expressed the activation markers. These data are representative of three independent experiments.
A second approach comparing the upregulation of early (CD69) and late (CD44) makers of CD8+ T-cell activation. This assay was performed ex vivo 30 days after the second immunization. Single-cell suspensions of SPL cells were simultaneously triple stained for CD3, CD8, and either CD69 or CD44. Compared to the control groups, in the Th-CTL lipopeptide immunized group both CD69 (Figure 3b) and CD44 (Figure 3c) were upregulated on gated CD8+ T cells. These findings demonstrate that virus specific CD8+ T cells with CTL activity were induced in the SPL after IVAG immunization with Th-CTL lipopeptide.
The local CD8+ T cells induced in vivo by IVAG immunization with lipopeptide are partially TLR2 dependent
We have previously demonstrated that, in vitro, antibody blocking of TLR-2, but not TLR-4, abrogates DC presentation of lipopeptide epitopes to T cells.33 Next, we assessed whether the lack of TLR-2 molecules will affect in vivo T-cell immunogenicity of Th-CTL lipopeptide delivered IVAG. Both gB498–505 and HSV-2-specific CD8+ T cells induced locally in the ILN by IVAG immunization with Th-CTL lipopeptide was compared in TLR2 knockout (TLR2−/−) H2b vs wild-type H2b mice. Four weeks after the last immunization, ILN-derived IFN-γ-producing CD8+ T cells that recognized HSV-infected H2b cells were assessed using IFN-γ-ELISpot assay. Higher number of HSV-2-specific IFN-γ-producing CD8+ T cells was detected by enzyme-linked immunosorbent spot (ELISpot) in wild-type H2b compared to TLR2−/− H2b mice (Figure 4a; wild-type vs TLR2−/−, P<0.002), consistent with TLR2's involvement.
Intravaginal (IVAG) immunization of helper-cytotoxic-T-lymphocyte chimeric epitopes (Th-CTL lipopeptide) induced interferon (IFN)-γ-producing T cells via toll-like receptor-2 signaling pathway. (a) Groups of wild-type B6 and TLR-2 knockout (TLR2−/−) H2b mice (n=5) were immunized IVAG with equimolar amounts of Th-CTL lipopeptide in saline. Twelve weeks after the second immunization, spleen (SPL)-derived CD8+ T cells in each group were stimulated in vitro for 5 days with glycoprotein B (gB)498–505-pulsed or heat-inactivated herpes simplex virus (HSV)-2-infected autologous H2b cells. The number of IFN-γ producing cells in each group of mice was determined by enzyme-linked immunosorbent spot (ELISpot) assay. Bars represent the mean values from quadruplicate wells in two independent experiments with ±s.e.m. Asterisk (*) indicates P<0.05. (b) SPL derived CD8+ T cells were stimulated in vitro with heat-inactivated virus pulsed Ag-presenting cells (APCs) and stained with a PE-labeled anti-CD8 mAb followed by an FITC-labeled HSV-gB498–505/H2-Kb tetramer. Cells were analyzed using a FACSCalibur with a total of 4 × 105 events collected for each point. Density plot shows the percentage of CD8+/Tetramer+ cells representative of two independent experiments. (c) Compares the in vitro proliferation of CFSE labeled gB498–505-specific CD8+ T cell in B6 and TLR-2 knockout (TLR2−/−) mice following IVAG immunization with Th-CTL lipopeptide (left) or saline alone (right).
Tetramer staining was performed to enumerate HSV-gB498–505-specific CD8+ T cells induced in wild-type H2b and TLR2−/− H2b mice 4 weeks following IVAG immunization with Th-CTL lipopeptide (Figure 4b). ILN-derived immune CD8+ T cells were isolated and restimulated in vitro with autologous HSV-2-infected SPL cells. Th-CTL lipopeptides induced a high percentage of HSV-gB498–505-specific CD8+ T cells (8.70±0.5%) in wild-type H2b mice. In contrast, a lower percentage of HSV-gB498–505-specific CD8+ T cells was detected in TLR2−/− mice (3.78±0.5%) (wild-type vs TLR2−/−, P=0.002).
In vivo proliferation of gB498–505-specific CD8+ T cells induced by IVAG/lipopeptide immunization, as detected in vivo by CFSE assay, was also lower in TLR2−/− mice compared to wild-type H2b mice (Figure 4c). Altogether, these results indicate that the mucosal T-cell immunogenicity of lipopeptides delivered IVAG is at least partially TLR-2 dependent.
IVAG immunization with Th-CTL lipopeptide, but not its Th-CTL peptide analog, protected against genital HSV-2 challenge
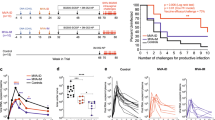
To evaluate whether IVAG immunization with Th-CTL lipopeptide conferred any protection against an intravaginal infection, mice received an IVAG HSV-2 challenge (2 × 104 pfu), 2 weeks following the second immunization. Mice immunized with lipopeptide showed significantly higher survival (90%) compared to mice immunized with the Th-CTL peptide control (only 60% of mice survived; P<0.005), with Th or CTL peptide alone (30% survived, respectively; P<0.005; Figure 5a). The pathology scores observed in the Th-CTL lipopeptide group were also much lower than all other groups (P=0.0002 for all; Figure 5b). Additionally, viral titers measured in the vaginal washes from days 5, 7, 9, and 11 postinfection showed that the Th-CTL lipopeptide group had significantly lower viral loads than the other groups (Figure 5c).
Protection against herpes simplex virus (HSV)-2 by intravaginal (IVAG) immunization of mice with helper-cytotoxic-T-lymphocyte chimeric epitopes (Th-CTL lipopeptide). Four groups of sex- and age-matched B6 mice (n=20) received an IVAG immunization with the Th-CTL lipopeptide, Th-CTL parental nonlipidated peptide, Th or CTL peptides alone. Two weeks after the second immunization (acute phase) mice were intravaginally challenged with HSV-2 (333, 2 × 104 pfu). (a) Survival following genital HSV-2 challenge. (b) Mean genital pathology scores following intravaginal HSV-2 challenge. Signs of disease were monitored daily and animals with pathology scores ≥4 were killed. (c) Average viral titer following HSV-2 challenge. Vaginal washes were collected daily following infection. Viral titers were measured by plaque assay on rabbit skin (RS) cell monolayers. Data show average virus titers in each group, detected on day 5, 7, 9, and 11 postinfection. The mean±s.e.m. of four mice per group are shown.
To assess the involvement of CD4+ and CD8+ T-cell subsets in the protection induced by IVAG immunization with the Th-CTL lipopeptide, in vivo depletion of either CD4+ or CD8+ T cells was performed in immunized mice before virus challenge. Depletion of CD8+ T cells, but not of CD4+ T cells, significantly abrogated the protection induced by immunization with the Th-CTL lipopeptide (P<0.005; Table 1), suggesting that in this system, CD8+ T cells are required for protection against lethal genital herpes.
Altogether, these results indicate that IVAG immunization with Th-CTL lipopeptides in the progestin-treated mouse model of genital herpes decreased virus replication at the site of infection and decreased overt signs of genital herpes disease. The results also suggest that CD8+ T-cell-mediated immunity was involved in the protection.
IVAG immunization with Th-CTL lipopeptide induced protective memory CD8+ T-cell responses
The longevity of the HSV-specific CD8+ T-cell response induced following IVAG immunization with Th-CTL lipopeptide was investigated 60 days after the final immunization. In DLNs which are the main location of the memory pool, Th-CTL lipopeptide immunization elicited memory CD8 T cells with cytotoxic activity against the HSV gB498–505 epitope, similar to that induced by HSV-2 TK− (Figure 6a). In addition, HSV specific memory CD8 T cells were detected by INF-γ ELISpot in DLN and SPL of both lipopeptide and HSV-2 TK− immunized mice (Figure 6b). The higher number of INF-γ-producing CD8+ T cells following HSV-2 TK− immunization is likely due to induction of responses to multiple HSV epitopes, in addition to gB498–505 epitope that is the unique CD8+ T-cell epitope in Th-CTL lipopeptide.
Intravaginal (IVAG) immunization with helper-cytotoxic-T-lymphocyte chimeric epitopes (Th-CTL) lipopeptide elicits protective memory CD8+ T-cell response. Three d groups of sex- and age-matched B6 mice (n=20) received an IVAG immunization with the Th-CTL lipopeptide, herpes simplex virus (HSV)-2 TK−−, or administered with phosphate-buffered saline (PBS) alone (Mock). Twelve weeks (memory phase) after the final immunization, local CD8+ T cells were harvested from draining lymph nodes (DLNs) of five B6 mice and stimulated for 5 days with HSV-infected autologous spleen cells. The cells were tested for (a) CTL activity following incubation with glycoprotein B (gB)498–505 peptide pulsed target cells by 51Cr release assay; (b) interferon (IFN)-γ-ELISpot assay. B6 mice (n=15) were first treated with Depo-Provera, to synchronize the estrous cycle, and then intravaginally challenged with HSV-2 (333, 2 × 104 pfu). (c) Survival. (d) Mean genital pathology scores and (e) Maximal viral titers in each group detected on day 5 postinfection were followed as in Figure 5.
To assess the protective ability of Th-CTL lipopeptide during the memory phase, mice were treated with Depo-Provera (DP) 60 days after the final immunization and then challenged intravaginally with 2 × 104 pfu of HSV-2, as above. Similar to HSV-2 TK− immunized mice, Th-CTL lipopeptide-immunized mice were more resistant to death, to genital disease and to virus replication than mock (phosphate-buffered saline; PBS)-immunized mice (Figure 6c–e). In vivo depletion of memory CD8+ T cells, but not of CD4+ T cells, significantly abrogated the protection against death induced by immunization with the Th-CTL lipopeptide (10% survived in CD8 depleted group, 80% survived in CD4 depleted group, 90% survived in nondepleted group (not shown). These results indicate that IVAG immunization with Th-CTL lipopeptide induced memory CD8 T cells and protection during the memory phase.
Absence of myeloid differentiation factor 88 abolishes HSV-specific CD8+ T-cell response and protection elicited by intravaginal lipopeptide immunization
To examine the contribution of MyD88, a critical adaptor protein shared by most of TLRs,35 in the immunogenicity of lipopeptides delivered intravaginally, groups of age-matched female MyD88−/− mice (n=10) and wild-type parental B6 mice (n=10) were immunized intravaginally either with Th-CTL lipopeptide or with live, attenuated HSV-2 (HSV-2 TK−; positive control). Negative control mice received PBS alone. Ten days after the second immunization, cells from DLNs were harvested and analyzed for HSV-specific CD8+ T-cell responses by IFN-γ-ELISpot assay as above. B6 mice developed significantly more HSV-specific IFN-γ-producing CD8+ T cells than MyD88−/− mice (P≤0.003; Figure 7a). In contrast, the apparent similar trend in HSV-2 TK− immunized mice did not reach significance. Thus, IVAG immunization with lipopeptide did not induce HSV-specific CD8+ T-cell responses in MyD88−/− mice, consistent with the role of MyD88 in the downstream signaling triggered by TLR-2 and lipopeptide (palmitic acid agonist) interactions.36
Myeloid differentiation factor 88 (MyD88) signaling is critical in helper-cytotoxic-T-lymphocyte chimeric epitopes (Th-CTL lipopeptide) immunogenicity and protective efficacy. (a) Groups of wild-type B6 and MyD88−/−-deficient mice (n=10) were immunized intravaginally (IVAG) with equimolar amounts of Th-CTL lipopeptide in saline. Two weeks after the second immunization, draining lymph nodes (DLNs) derived CD8+ T cells from each group were stimulated in vitro for 5 days with glycoprotein B (gB)498–505-pulsed or heat-inactivated herpes simplex virus (HSV)-2-infected autologous H2b cells. The numbers of interferon (IFN)-γ-producing cells were measured by enzyme-linked immunosorbent spot (ELISpot) assay. Asterisk (*) indicates P<0.003. (b) Survival. (c) Mean genital pathology scores and (d) Viral titers in each group detected on day 5 postinfection were followed as in Figures 5 and 6.
In an additional experiment, mice immunized as above, received DP treatment and were challenged intravaginally with 2 × 104 pfu of HSV-2 10 days after the final immunization. MyD88−/− mice immunized with lipopeptide had increased death and developed more genital disease compared to B6 mice (Figure 7b and c), whereas HSV-2 TK− immunization provided similar protection against both genital disease and death in wild-type and MyD88−/− mice. In addition, lipopeptide immunized MyD88−/− mice had significantly higher virus titers in their genital secretions on day 5 compared to lipopeptide immunized B6 mice (P≤0.005; Figure 7d). As expected, wild-type and MyD88−/− mice immunized with HSV-2 TK− had similar reduced virus titers compared to control nonimmunized wild-type and MyD88−/− mice (Mock). Together, these results strongly suggest that MyD88 is important for the ability of Th-CTL lipopeptide to induce mucosal immunity following intravaginal immunization.
Discussion
Induction of pathogen-specific T cells through an IVAG immunization with nonreplicating, nontoxic and self-adjuvanting subunit vaccines is seen as an ideal approach to prevent the transmission or limit the severity of sexually transmitted infectious diseases. In this study, we demonstrated that TLR-2 targeting by a lipopeptide vaccine administered into the GT-induced protective HSV-2-specific CD8+ T-cell responses. Mice immunized IVAG with the herpes lipopeptide showed higher survival and lower pathology scores following genital HSV-2 challenge than mice immunized with nonlipidated peptide analog. Moreover, vaginal viral replication was reduced in the lipopeptide-vaccinated group. These findings clearly demonstrate that, although the GT has been considered a poor inductive site for immunization with nonreplicating subunit vaccines,6 IVAG delivery of a self-adjuvanting Th-CTL lipopeptide stimulates both local and systemic HSV-specific protective T-cell response. The finding merits further evaluation of the lipopeptide/IVAG vaccine strategy against other sexually transmitted infections.
Mucosal surfaces constitute an impressive first-line defense system that is frequently exposed to an array of exogenous Ags.29, 37, 38 Mucosa of the female and male GT are the portals of entry for many sexually transmitted infectious diseases of viral, bacterial, fungal, and parasitic origin.14, 39 The mucosal immune system is largely separate and distinct from the systemic immune system.31 Several studies have shown that there is also compartmentalization within the mucosal immune system itself, so that mucosal immunization induces stronger immune responses at, and adjacent to the site of induction, compared to distant sites.14, 29, 31, 37, 38, 39 Although the GT is considered a component of the mucosal immune system, it displays several distinct features not shared by other typical mucosal immune systems.6, 40 For example, GT tissues lack inductive mucosal sites analogous to nasal associated lymphoid tissue6, 40 or intestinal Peyer's patches.38 The female GT does however have the ability to initiate an immune response against infection by a variety of sexually transmitted pathogens, such as HSV-2 and Neisseria gonorrhoeae.14, 29, 37, 39 The GT lacks organized lymphoid structures resembling the follicules of the conjunctiva where the ocular mucosal immune responses are initiated and disseminated to other effector sites.6, 40, 41 Consequently, local T-cell responses stimulated in the GT by subunit vaccine (e.g., recombinant proteins or peptide epitopes) have been weak or absent, and repeated IVAG immunizations result in minimal immune responses.12 Several studies of GT immunization have shown that administration of nonreplicating antigens into the vagina can result in the generation of specific immunity.13, 40, 42 However, these responses were rather modest and were not disseminated either to other mucosal sites or to the systemic compartment.
Thus, during the last two decades, the development of herpes vaccines delivered into GT has almost exclusively been based on live attenuated vaccines.5, 12, 14 The focus on live vaccines also comes because an efficient immunization in the GT likely requires strategies capable of stimulating local CD8+ CTLs, i.e., the vaccine must deliver antigen to endogenous MHC class I pathway. However, safety concerns are often voiced regarding live GT vaccination. Molecularly defined antigenic formulations for IVAG vaccination offer a noninvasive means of enhancing the immune response against target antigens and thus may offer an approach in human immunization protocols in which CD8+ T-cell induction is critical.29, 30, 43 Reports showed that subunit vaccine delivered with CpG mucosal adjuvant, a TLR-9 agonist, induced protective immunity in mice against vaginal challenge with HSV-2.,17, 44 Most lipopeptide vaccine approaches have been tested parenterally4, 45, 46, 47 whereas a few reports showed enhancement of mucosal immune responses following intranasal delivery.4, 48 We previously demonstrated that intranasally administered human cytomegalovirus and malaria-derived lipopeptide epitopes induced both mucosal and systemic B and T-cell responses.4, 29, 30 Studies have also reported that mucosal adjuvant macrophage-activating lipopeptide-2 directly stimulates B cells via TLR-2 without the need of accessory cells.49
This study is the first to demonstrate that lipopeptide-delivered IVAG efficiently stimulates HSV-specific local and systemic CD8+ T cells via TLR-2. Importantly, the induced CD8+ T cells interfered with virus replication in the GT and protected mice from most overt signs of herpes disease. The efficacy of lipopeptides as nonreplicating, nontoxic, and self-adjuvanting anti-HSV vaccines has clearly demonstrated that protective antiviral immunity can be achieved by non-live virus strategies. The study also showed that IVAG immunization with Th-CTL lipopeptide can induce local memory CD8+ T cells in the draining lymph nodes and decrease death, disease progression, HSV-2 titers in the vagina. Thus, local HSV-specific CD8+ T cells are likely important immune effectors in viral clearance from the vaginal mucosa.
A major goal of HSV-2 vaccine development is the induction of mucosal immune responses able to stop or reduce viral infection directly at the portal of entry. Shortcomings in developing an effective immunization strategy against genital herpes include an imperative requirement for a safe delivery system.20 In most cases, unmodified nonvectorized peptide epitopes fail to elicit specific T cells, unless they are attached to a carrier protein or delivered with a strong adjuvant.6 Often, the delivery of the peptides in this manner is unsafe and/or promotes Th2 types of responses,29, 30, 43 which would not be expected to provide optimal protection against genital herpes.50, 51, 52, 53 Extending peptide epitopes with a TLR-2 agonist (e.g., palmitic acid) generates cell-mediated responses in the absence of external adjuvant, apart from the lipid moiety itself, and offers a safer immunogenic formulation than commonly employed external adjuvants.4, 29, 30 Lipopeptide vaccines are potent, safe and practical for use in humans.4, 29, 30 Lipopeptide formulations are easily manufactured, can be sterilized by filtration, and are compatible with all antigens tested to date.4, 29, 30 Lipopeptide vaccines delivered parenterally have been shown in clinical trials to be potent stimulators of CD4+ T cells, CD8+ T cells, and Ab responses.33, 54 Physicochemical properties and toxicology studies in animal models and in two Phase I studies have established the safety of HIV and malaria lipopeptide vaccines.33, 54, 55 The present report focused on herpes lipopeptides and demonstrated their ability, when delivered in saline via the IVAG route, to induce CD8+ T-cell-dependent protective immunity against genital herpes. As HSV-2 infection, usually acquired through genital contact, invades human mucosa, the use of an IVAG immunization may offer a safer and more effective route for a genital herpes vaccine.42, 56
A recent study showed generation of a protective immunity against genital herpes after IVAG immunization with the HSV-2 recombinant gB mixed with CpG external adjuvant.12 Here we showed that a single immunodominant CD8+ T-cell epitope from gB delivered IVAG in the form of a self-adjuvanting lipopeptide also generates protective immunity. The use of different adjuvants and Ags likely explains differences between the two studies regarding the effector mechanism of protection. Thus, in our study we found that local CD8+ T cells are key effectors in the protection against genital herpes, whereas the study above attributed the protection mainly to IgG/IgA antibodies. As neutralizing antibody does not appear to play a major role in protection against HSV in humans,57 the use of lipopeptide, a TLR-2 agonist that appeared to simulate mucosal cellular immunity may be a preferred adjuvant over the TLR-9 agonist CpG, that appeared to simulate mostly mucosal humoral immunity. In addition, CpG causes toxicity in both animals39, 58, 59, 60 and humans,61, 62 whereas the lipid moiety used here appears safer in both animals29, 30, 33, 46, 63, 64, 65 and in recent clinical trials.47, 66, 67 Molecularly defined epitope-based mucosal vaccines capable of inducing anti-HSV immune responses, in a manner compatible with human delivery, are limited. Few molecules achieve this target without utilizing external mucosal immunoadjuvant. The lipopeptide vaccine described here combines minimal sequences of the HSV-gB CD8+ CTL epitope (gB498–505) along with a Th epitope (PADRE) covalently linked to a TLR-2 agonist (the palmitic acid moiety). The Th-CTL lipopeptide acts as a self-adjuvanting immunogen following IVAG delivery, facilitating the generation of an HSV-specific CD8+ T-cell response via the TLR-2-MyD88 pathway. We should also give emphasis to our recent observation68 that HSV gB protein contains “symptomatic” T-cell epitopes, which are potentially harmful rather than protective against genital herpes disease. Thus, epitope-based vaccines that exclude “symptomatic” epitopes should be preferable to whole protein based vaccines that likely include both protective and harmful epitopes.68
The cellular and molecular mechanism underlying the strong T-cell immunogenicity of lipopeptides when delivered mucosally has yet to be fully elucidated. In this study, we have demonstrated that, in vivo, TLR2 was involved in T-cell immunogenicity of lipopeptide delivered IVAG. This implies that lipopeptide might engage TLR2 directly on CD8+ T cells and lower the threshold for optimal antigen-induced T-cell activation. This phenomenon has been recently reported.69 DCs are essential component of the mucosal immune system. They are the professional Ag-capturing and Ag-presenting cells (APCs) with the unique ability of priming naive CD4+ as well as CD8+ T cells.33 It has become increasingly clear that induction and modulation of mucosal immunity against infectious pathogens requires immunogenic formulations that allow efficient targeting and maturation of DCs.4, 70 In addition, DCs in the GT express high levels of TLR-2 and are important effector cells bridging the innate and acquired immune responses during HSV-2 vaginal clearance.70 An essential feature of lipopeptide immunogenicity is the lipid component, which is thought to enhance Ag-uptake, to redirect the peptide epitope to either exogenous or endogenous pathways of antigen presentation and to induce DCs maturation allowing efficient priming of naive T cells.4, 29, 30, 33 We have previously demonstrated that lipopeptides are taken up predominantly by immature DCs.46, 71, 72 This appeared to increase cell surface expression of MHC and costimulatory molecules and produced proinflammatory cytokines, thus providing efficient T-cell stimulation.34 Altogether, the present findings of TLR-2 requirement for IVAG lipopeptide immunogenicity provide further insight into the cellular and molecular mechanisms behind the potent CD8+ T-cell immunogenicity of lipopeptides and confirm recent reports from our and other groups.4, 29, 30, 33, 70
DCs are responsible for the priming of anti-HSV cell-mediated immunity at the systemic as well as at the mucosal level.1 We previously reported that DCs are the predominant APCs, besides the monocytes/macrophages, involved in the uptake of lipopeptide Ag.30 DCs are a heterogeneous population of antigen-presenting cells, of which conventional DCs and plasmacytoid DCs are the main subsets. Like DC subsets in the central lymphoid organs, DC subsets in the vaginal mucosa also exert specific functions that can be associated with distinct expression of endocytic receptors and cell-surface molecules. In recent years, DC populations are increasingly split up into a seemingly endless number of defined subpopulations. Understanding the phenotypic and functional role of different DC subsets during mucosal immunization is essential for a deeper understanding of the immune response to pathogens such as HSV-2. The fine-tuned balance that exists between the various functions of vaginal DC subsets, which are necessary for maintaining immune homeostasis in the vagina, remain to be fully elucidated. However, IVAG mucosal infection or immunization can profoundly alter the functions of steady-state DC subsets and recruit inflammatory type DCs to the vagina. We hypothesize that on IVAG lipopeptide immunization, the plasmacytoid DCs recruited to the vaginal mucosa are the main APCs targets of our lipopeptide vaccine strategy, as has been recently shown in other systems.1 Therefore, it will be important to characterize these DC subsets in vaginal mucosa and DLNs and assess their role during IVAG immunization with Th-CTL lipopeptide.
Interaction of lipopeptides with DCs—the principal immune-competent cells to encounter antigens within mucosal membranes, triggered their phenotypic and functional maturation.4, 33 In addition, we showed that, in vitro, presentation of lipopeptide by DCs to T cells involved interaction with TLR-2 on the cell surface of DCs.33 It has been reported that the tripalmitoyl lipopeptide Pam(3)CysSK(4) (Pam), interacts directly with mouse T cells via TLR-2.69 Such interaction induced a costimulation and produced an increase in T-cell proliferation and survival associated with sustained CD25 expression and enhanced expression of Bcl-xL anti-apoptotic protein. However, whether the tri-palmitoyl lipopeptide used in this study acts in vivo to induce T cells indirectly (by interaction with DCs) or by direct interaction with T cells remains to be determined. Cottalorda has recently reported that in vivo TLR-2 engagement on T cells lowers the activation threshold for costimulatory signals delivered by APC.69 Based on these results and our own report on the effect of lipopeptide on the phenotypic and functional maturation of DCs, 4, 29, 30, 33 it is likely that the strong mucosal immunogenicity of lipopeptides involves interaction of the lipid moiety with both DCs and T cells. The cellular mechanisms underlying the mucosal immunogenicity of these lipopeptides are currently being investigated in our laboratory.
MyD88 is a critical adaptor protein shared by most TLRs, including TLR-2.35 In this study, we have found that MyD88 is important for mucosal immunogenicity and protective efficacy of Th-CTL lipopeptide. Compared to wild-type B6 mice, MyD88−/− mice develop significantly lower HSV-specific CD8+ T-cell responses following intravaginal immunization with Th-CTL lipopeptide. In addition, Th-CTL lipopeptide immunized MyD88−/− mice remained highly susceptible to genital herpes. Thus, following vaginal challenge with HSV-2, MyD88−/− mice had higher vaginal HSV-2 titers; and faster genital disease progression compared to wild-type B6 mice. To our knowledge, this is the first evidence that the adaptor protein MyD88 is required for induction of HSV-specific CD8+ T-cell immunity following vaginal immunization with a lipopeptide. These findings have implications for the development of novel immunization approaches to generate adaptive mucosal immunity in the female GT against herpes. These findings also point to the important role of the TLR-2/MyD88 signaling pathway in mucosal immunity induced by Th-CTL lipopeptides. However, they do not exclude the involvement of other intracellular signaling pathways in the adjuvanticity of palmitic acid moiety and in the lipopeptide mucosal immunogenicity. The intracellular pathways underlying TLR-2 signaling following IVAG immunization with lipopeptides are currently being investigated in our laboratory.
The chemical attachment of the TLR-2 agonist to the Th-CTL peptide backbone might ensures its uptake by TLR-2-expressing cells such as DC and epithelial cells within the GT mucosa, which then assist antigen processing and full activation of local T cells. The synthetic approach makes it possible to optimize the various components of future candidate Th-CTL lipopeptide vaccines, bearing recently identified human Th and CTL epitopes,73 by structure–activity relationship studies. In this respect, proper design of the three-component vaccine is essential, because it has been shown that a compound composed of the inferior adjuvant Pam3Cys (which lacks the important tetralysine moiety) and a human Th epitope elicits low immune response (reviewed in Moyle and Toth48 and BenMohamed et al.4). Furthermore, we found here that covalent attachment of the three components is required for optimal antigenic responses in the GT. Probably, the lipid moiety may facilitate proper presentation and retention of the Th-CTL peptide within the GT.
Both CD4+ and CD8+ T cells appear to be the major effectors in controlling genital HSV-2 infection and disease.68, 73, 74, 75, 76, 77 Even though clearance of HSV-2 from recurrent genital lesions correlates with the infiltration of both HSV-2-specific CD4+ and CD8+ T cells,78, 79 the CD4+ T cells infiltrate first and are associated, time-wise, with a drop in infectious virus titer within the vaginal lesions.80, 81 CD4+ T cells, directed against envelope and capsid proteins are thought to be among the main mediators of protective immunity during recurrent genital herpes.82, 83, 84, 85
Besides CD4+ T cells, several lines of evidence, in both animal models and humans, also support a critical role for CD8+ T cells in the control of HSV infection and in surveillance function that limits virus reactivation.78, 79, 86, 87, 88, 89 Local infiltration of HSV-specific CD8+ CTL correlates with clearance of infectious virus particles in human recurrent genital herpes.78, 79 HSV-2-specific CD8+ T cells persistently infiltrate healed genital herpes lesions.52 In mice, HSV-2-specific CD8+ T cells infiltrate acute and latently infected ganglia and mediate control over viral reactivation in an IFN-γ-dependent manner.90 Depletion of CD8+ T cells impairs clearance of virus from sensory neurons, whereas adoptive transfer of TCR transgenic CD8+ T cells, specific for gB498–505 epitope, into naive mice lacking other components of adaptive immunity results in viral clearance.91, 92 In this study, we used a totally synthetic Th-CTL lipopeptide that incorporates the immunodominat gB498–505 epitope along with a CD4+ T-cell epitope (Pan DR or PADRE epitope) and demonstrated a strong protection against genital herpes following IVAG delivery. Although the protective immunity induced by this self-adjuvanting formulation is CD8+ T-cell-dependent, coinduction of CD4+ T cells and the lipid component are critical for the establishment of the protective CD8+ T cells. Indeed, the corresponding Th-CTL peptide analog without the lipid or, the CTL lipopeptide alone, or the Th lipopeptide alone failed to induce the protective immunity (not shown). CD4+ T cells fulfill their role of T helper in providing help for the establishment of CD8+ T-cell memory during initial antigen priming.37 It is also possible that the lipid will have a role at the early phase of immune induction through its ability to mature the priming DC in a TLR-2-dependent manner, as we recently reported.4, 29, 30 The possibility exists that lipopeptide induced local T cells limit HSV-2 replication at the initial site of infection, the vaginal mucosa, and thus prevents the infection from innervating neurons.56 In addition, immunization with lipopeptide improved the magnitude of IFN-γ production, a type of response that has been linked to protection from IVAG infection with HSV-2.93 Together, these result suggest that the mechanisms underlying the protection induced by lipopeptides may involve local CD8+ T cells, which secrete cytokines such as IFN-γ which limit the establishment of viral infection in the vaginal tract and/or its spread to dorsal ganglia and the central nervous system.94 IFN-γ may also be essential in increasing the effectiveness of T-cell-dependent virus clearance mechanisms by upregulating MHC class I expression on HSV-infected cells to allow better recognition and lysis by CD8+ T cells.33, 95 That immunization with lipopeptide generates IFN-γ production is consistent with our studies and previous reports demonstrating that induction of T-cell responses by gD lipopeptides correlates with viral protection.33
In conclusion, this study illustrates the vaccine potential of a herpes lipopeptide against genital HSV-2 infection on an IVAG delivery in the absence of external adjuvant. The results demonstrate that the protective efficacy of this formulation is associated with the induction of CD8+ T-cell responses. Lipopeptides engineered in this study using the chimoselective ligation are simple to produce, pure under GMP conditions and produce enhanced local and systemic immunogenicity without the need for external mucosal adjuvant. They offer a needle-free and relatively low-cost route to provide material for human herpes vaccination trials.
Methods
Mice. TLR2 knockout (TLR2−/−) and MyD88 deficient mice (MyD88−/−), 4–5 weeks old, were provided by Shizuo Akira (Osaka University, Osaka, Japan), and were on the C57BL/6 background. Female C57BL/6 (H2b) mice, 4–5 weeks old, were purchased from the Jackson Laboratory (Bar Harbor, ME). Animal studies conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health.
Virus and cell lines. HSV-2 strain 333 was obtained from Dr Straus (NIH). The live attenuated thymidine kinase-deficient HSV-2 (HSV-2 TK−), was provided by James R. Smiley and Lynda A. Morrison. Triple plaque-purified HSV-2 was prepared as we described previously.4, 41, 50, 63 Rabbit skin cells, used to prepare virus stocks and to culture virus from vaginal swabs, were grown in Eagle's minimum essential medium supplemented with 5% fetal calf serum (InvitrogenGibco, Grand Island, NY). Heat-killed virus was made by heating virus solution at 100 °C for 5 min. HSV inactivation was confirmed by the inability to produce plaques when tested on Vero cells, as we described.41, 71
Peptides and lipopeptides. The structure of the totally synthetic palmitoyl-tailed Th-CTL lipopeptides used in this study is shown in Figure 8 and has been previously described.34 Briefly, as a model antigen, the HSV-gB CD8+ CTL epitope (gB498–505) was synthesized in line with the PADRE, a universal CD4+ Th epitope. The peptide backbone, composed solely of both epitopes, was extended by N-terminal attachment of three ((PAM)3) palmitoyl lysines.
Illustration of the helper-cytotoxic-T-lymphocyte chimeric epitopes (Th-CTL lipopeptide) used in this study. (a) Structure of the lipidated Th-CTL lipopeptide (LIPO). The C-terminal region of the Pan DR peptide (PADRE; center) was fused to the N-terminal region of the herpes simplex virus (HSV) glycoprotein B (gB)498–505 target peptide (right). The resulting Th-CTL chimeric peptide backbone, was modified by N-terminal addition of three palmitoyl lysines (TLR-2 agonist) shown in the box. The amino acid sequences of CD4+ T helper epitope (Th or PADRE) and CD8+ T-cell epitope (CTL or gB498–505) are indicated in a single-letter code. dA, L-alanine; Cha, L-cyclohexyl alanine; Ahx, aminocaproic acid. (b) Structure of nonlipidated Th-CTL peptide analog (PEP).
Immunizations and virus challenge. Five days before the first immunization and before challenge, to synchronize the estrous cycle at the progesterone-dominated stage and to facilitate uniform infection with HSV-2,96 mice received three subcutaneous injections of 3 mg of DP (or medroxyprogesterone acetate; Upjohn Company, Kalamazoo, MI). Mice were immunized IVAG with equimolar amount (100 μM) of Th-CTL lipopeptide, Th-CTL nonlipidated peptide control, Th peptide (PADRE), or CTL peptide alone in PBS on days 0 and 21. In some experiments, mice were immunized IVAG with the live attenuated HSV-2 TK− , as previously described.97
A preliminary experiment was conducted to determine the LD50 in mice of HSV-2 by intravaginal route. Five days following DP administration, the animals were challenged by IVAG inoculation of 2 × 104 pfu of HSV-2 in 20 μl as described previously.42, 56
Three weeks following the second immunization, mice were anesthetized by injectable anesthetic (ketamine–xylazine (0.75:0.25 ml)) given intraperitoneally, placed on their backs, and infected intravaginally with 2 × 104 pfu of HSV-2 in a total volume of 10 μl. Mice were kept on their backs under the influence of anesthesia for 45 min to 1 h to allow infection.
Mice were anesthetized with sodium pentobarbital before virus inoculation, preswabbed with a sterile calcium alginate swab, and inoculated by instillation of virus suspension into the vaginal lumen. Mice were kept on their backs under the influence of anesthesia for 30 min to allow vaginal infection with the inoculum.
ELISpot assay. SPL cells were cultured in 24-well plates for 5 days in a humidified 5% CO2 atmosphere with HSV-gB498–505 peptide alone (10 μg/ml), the irrelevant OVA257–264 CD8+ T-cell peptide (10 μg/ml), autologous HSV-2 infected stimulator cells, or mock-infected stimulator cells, and subsequently analyzed in an IFN-γ-ELISPOT assay.95 IFN-γ-ELISpot assays were performed using the mouse IFN-γ-ELISPOT mAb pair (BD Pharmingen, San Diego, CA). Briefly, on day 4, 96-well multi-screen-IP plates were coated over night at 4 °C with 100 μl (1:250) of anti-IFN-γ capture mAb. Plates were then blocked with RPMI-1640 medium supplemented with 10% fetal calf serum for 2 h at room temperature. 5 × 104 cells/well were added in triplicate to mAb coated plates and incubated for 18–24 h at 37 °C, 5% CO2. Plates were then washed with PBS, supplemented with a detection peroxidase-labeled antibody followed by a substrate according to the manufacturer's instructions. The developed spots were counted under a light microscope.
Flow cytometry. Standard flow cytometry was employed, as we previously described.4, 29, 30 Various cell surface markers were assessed using FITC-anti-mouse CD4 mAb (GK1.5), FITC-anti-mouse CD8a mAb (53–6.7) (Pharmingen, San Diego, CA) and PE-labeled HSV-gB498–505/H2-Kb tetramer (NIH Tetramer Facility). IgG isotype-matched control Abs were used in all experiments. After staining, cells were washed and fixed in 1% buffered paraformaldehyde before being acquired on a Becton Dickinson FACSCalibur (Mountain View, CA). For each sample, 20,000 events were acquired on a FACSCalibur and analyzed with CellQuest software, on an integrated Macintosh G4 (Becton Dickinson, San Jose, CA).
In vitro CTL activity. Spleen derived immune CD8+ T cells (5 × 106) were restimulated in vitro with HSV-gB498–505 target peptide (5 μg/ml)-pulsed syngeneic-irradiated T-cell-depleted 1 × 106 splenocytes (2,500 rad from a 137Cs source) and irradiated 1 × 106 EL-4 cells (3,000 rad from a 137Cs source), as we previously described.4, 29, 30, 63 CTL activity was assessed by a standard 4-h 51Cr release assay against EL4 target cells loaded with 10 μM of HSV-gB498–505 target peptide or infected with heat-inactivated HSV-2 (multiplicity of infection=3) as previously described37, 63, 95. After 5 days of culture, effector CD8+ cells were mixed at 1, 3, 10, 30, or 100 effector–target (E:T) ratios, with 51Cr-labeled EL4 cells for 4 h. Maximum release of 51Cr was determined by adding 5% Triton X-100 to 51Cr-labeled EL4 cells. Spontaneous release (<10% of total release), was determined by incubating target EL4 cells with medium alone. The percentage of specific lysis was calculated as follows: 100 × ((experimental release × spontaneous release)/(maximum release−spontaneous release)).
In vivo CTL assay. The in vivo CTL assay was carried out as previously reported.98 SPL cells from naive B6 mice were used as target cells and equally split into two populations. One was pulsed with 2.5 μg gB498–505 peptide for 1 h at 37 °C and then labeled with a high concentration (2.5 μm) of CFSE. The other nonpulsed population was labeled with a low concentration of CFSE (0.25 μm). Equal number of cells from each population (107 cells) was mixed together and adoptively transferred i.v. into naive and immunized B6 mice. In some experiments, splenocytes were collected either 1 or 4 h after adoptive transfer from recipient mice, erythyrocytes were lysed, and cell suspensions were analyzed by FACS. Each population was distinguished by their respective fluorescence intensity. Assuming that the number of peptide-pulsed cells injected is equivalent to the number of no peptide-pulsed cells injected, the percentage of killing of target cells in uninfected animals was determined as:
((Percentage of CFSElow−percentage of CFSEhigh) × 100)/Percentage CFSElow.
The percentage killing of target cells in infected animals was calculated as:
Ratio=(Percentage of CFSElow/percentage of CFSEhigh).
Percentage specific lysis=(1−(ratio uninfected/ratio infected) × 100).
In vivo depletion of CD4+ and CD8+ T cells. In some experiments, beginning 21 days after the second dose of peptide vaccine, mice were i.p. injected with six doses of 100 μl of PBS containing mAb GK1.5 (anti-CD4), a mAb 2.43 (anti-CD8) or hamster immunoglobulin treated control (NCCC, Minneapolis, MN) on days −7, −1, 0, 2, and 5 postinfection. Depletion of T cells was assessed by flow cytometric analysis of splenocytes at the end of the experiment (days 12–13 postinoculation).
Monitoring virus replication. To quantify vaginal HSV-2, the vaginal canal of immunized and control mice were swabbed once daily (days 1–10 postinfection) with a Dacron swab and each swab placed in a 75-mm culture tube containing 0.5 ml of media. 100 μl aliquots of 10-fold serial dilutions were placed on confluent monolayer of rabbit skin cells in six-well plates, incubated at 37 °C for 1 h and overlaid with medium containing 1% methylcellulose. The plates were incubated at 37 °C for 3 days, stained with 1% crystal violet, and the viral plaques were counted.
Genital pathology. Genital pathology following infection with HSV-2 was monitored daily and was scored on a five-point scale: 0, no apparent infection; 1, slight redness of external vagina; 2, swelling and redness of external vagina; 3, severe swelling and redness of both vagina and surrounding tissue and hair loss in genital area; 4, genital ulceration with severe redness, swelling, and hair loss of genital and surrounding tissue; 5, severe genital ulceration extending to surrounding tissue. Animals were killed after stage 4. For the purpose of these studies animals that did not develop symptoms were defined as infected if virus was isolated from the vaginal swab specimens collected on day 2 after inoculation.11, 12, 39, 93
Statistical analysis. Figures represent data from two or three independent experiments. The data was expressed as the mean±standard error of the mean and were compared by Student's t-test using a STATVIEW II statistical program (Abacus Concepts, Berkeley, CA) and by two-way ANOVA using Graphpad Prism 4. Differences were considered significant when P<0.05. All P values were two-tailed unless stated otherwise.
Disclosure
The authors declared no conflict of interest.
References
Yoneyama, H. et al. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J. Exp. Med. 202, 425–435 (2005).
Tigges, M.A. et al. Human CD8+ herpes simplex virus-specific cytotoxic T-lymphocyte clones recognize diverse virion protein antigens. J. Virol. 66, 1622–1634 (1992).
Koelle, D.M. et al. Herpes simplex virus infection of human fibroblasts and keratinocytes inhibits recognition by cloned CD8+ cytotoxic T lymphocytes. J. Clin. Invest. 91, 961–968 (1993).
BenMohamed, L., Wechsler, S.L. & Nesburn, A.B. Lipopeptide vaccines—yesterday, today, and tomorrow. Lancet. Infect. Dis. 2, 425–431 (2002).
Koelle, D.M. Vaccines for herpes simplex virus infections. Curr. Opin. Investig. Drugs 7, 136–141 (2006).
Russell, M.W. Immunization for protection of the reproductive tract: a review. Am. J. Reprod. Immunol. 47, 265–268 (2002).
Langenberg, A.G., Corey, L., Ashley, R.L., Leong, W.P. & Straus, S.E. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. N. Engl. J. Med. 341, 1432–1438 (1999).
Celum, C., Levine, R., Weaver, M. & Wald, A. Genital herpes and human immunodeficiency virus: double trouble. Bull. World Health Organ. 82, 447–453 (2004).
Kaul, R. et al. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. J. Reprod. Immunol. 77, 32–40 (2008).
MasCasullo, V., Fam, E., Keller, M.J. & Herold, B.C. Role of mucosal immunity in preventing genital herpes infection. Viral. Immunol. 18, 595–606 (2005).
Milligan, G.N., Dudley-McClain, K.L., Chu, C.F. & Young, C.G. Efficacy of genital T cell responses to herpes simplex virus type 2 resulting from immunization of the nasal mucosa. Virology 318, 507–515 (2004).
Kwant, A. & Rosenthal, K.L. Intravaginal immunization with viral subunit protein plus CpG oligodeoxynucleotides induces protective immunity against HSV-2. Vaccine 22, 3098–3104 (2004).
Hamajima, K. et al. Systemic and mucosal immune responses in mice after rectal and vaginal immunization with HIV-DNA vaccine. Clin. Immunol. 102, 12–18 (2002).
Gallichan, W.S. & Rosenthal, K.L. Long-term immunity and protection against herpes simplex virus type 2 in the murine female genital tract after mucosal but not systemic immunization. J. Infect. Dis. 177, 1155–1161 (1998).
Schleiss, M.R., Bourne, N., Jensen, N.J., Bravo, F. & Bernstein, D.I. Immunogenicity evaluation of DNA vaccines that target guinea pig cytomegalovirus proteins glycoprotein B and UL83. Viral. Immunol. 13, 155–167 (2000).
Gherardi, M.M., Perez-Jimenez, E., Najera, J.L. & Esteban, M. Induction of HIV immunity in the genital tract after intranasal delivery of a MVA vector: enhanced immunogenicity after DNA prime-modified vaccinia virus Ankara boost immunization schedule. J. Immunol. 172, 6209–6220 (2004).
Tengvall, S., Lundqvist, A., Eisenberg, R.J., Cohen, G.H. & Harandi, A.M. Mucosal administration of CpG oligodeoxynucleotide elicits strong CC and CXC chemokine responses in the vagina and serves as a potent Th1-tilting adjuvant for recombinant gD2 protein vaccination against genital herpes. J. Virol. 80, 5283–5291 (2006).
Rosenthal, K.L. & Gallichan, W.S. Challenges for vaccination against sexually-transmitted diseases: induction and long-term maintenance of mucosal immune responses in the female genital tract. Semin. Immunol. 9, 303–314 (1997).
Toka, F.N., Pack, C.D. & Rouse, B.T. Molecular adjuvants for mucosal immunity. Immunol. Rev. 199, 100–112 (2004).
Stanberry, L.R. Clinical trials of prophylactic and therapeutic herpes simplex virus vaccines. Herpes 11 (Suppl 3), 161A–169A (2004).
Mestecky, J., Moldoveanu, Z. & Russell, M.W. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am. J. Reprod. Immunol. 53, 208–214 (2005).
Moldoveanu, Z., Huang, W.Q., Kulhavy, R., Pate, M.S. & Mestecky, J. Human male genital tract secretions: both mucosal and systemic immune compartments contribute to the humoral immunity. J. Immunol. 175, 4127–4136 (2005).
Gill, N., Davies, E.J. & Ashkar, A.A. The role of toll-like receptor ligands/agonists in protection against genital HSV-2 infection. Am. J. Reprod. Immunol. 59, 35–43 (2008).
Herbst-Kralovetz, M.M. et al. Quantification and comparison of toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia. Am. J. Reprod. Immunol. 59, 212–224 (2008).
Soboll, G., Schaefer, T.M. & Wira, C.R. Effect of toll-like receptor (TLR) agonists on TLR and microbicide expression in uterine and vaginal tissues of the mouse. Am. J. Reprod. Immunol. 55, 434–446 (2006).
Zariffard, M.R. et al. Induction of tumor necrosis factor- alpha secretion and toll-like receptor 2 and 4 mRNA expression by genital mucosal fluids from women with bacterial vaginosis. J. Infect. Dis. 191, 1913–1921 (2005).
Lund, J.M., Linehan, M.M., Iijima, N. & Iwasaki, A. Cutting edge: plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J. Immunol. 177, 7510–7514 (2006).
Fichorova, R.N., Cronin, A.O., Lien, E., Anderson, D.J. & Ingalls, R.R. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of toll-like receptor 4-mediated signaling. J. Immunol. 168, 2424–2432 (2002).
BenMohamed, L., Krishnan, R., Auge, C., Primus, J.F. & Diamond, D.J. Intranasal administration of a synthetic lipopeptide without adjuvant induces systemic immune responses. Immunology 106, 113–121 (2002).
BenMohamed, L. et al. Systemic immune responses induced by mucosal administration of lipopeptides without adjuvant. Eur. J. Immunol. 32, 2274–2281 (2002).
Borsutzky, S. et al. Efficient systemic and mucosal responses against the HIV-1 Tat protein by prime/boost vaccination using the lipopeptide MALP-2 as adjuvant. Vaccine 24, 2049–2056 (2006).
Batzloff, M.R., Hartas, J., Zeng, W., Jackson, D.C. & Good, M.F. Intranasal vaccination with a lipopeptide containing a conformationally constrained conserved minimal peptide, a universal T cell epitope, and a self-adjuvanting lipid protects mice from group a streptococcus challenge and reduces throat colonization. J. Infect. Dis. 194, 325–330 (2006).
Zhu, X., Ramos, T.V., Gras-Masse, H., Kaplan, B.E. & BenMohamed, L. Lipopeptide epitopes extended by Ne-Palmitoyl lysine moiety increases uptake and maturation of dendritic cell through a toll-like receptor 2 pathway and triggers a Th1-dependent protective immunity. Eur. J. Immunol. 34, 1142–1149 (2004).
Zhang, X. et al. Th-cytotoxic T-lymphocyte chimeric epitopes extended by Nepsilon-palmitoyl lysines induce herpes simplex virus type 1-specific effector CD8+ Tc1 responses and protect against ocular infection. J. Virol. 79, 15289–15301 (2005).
Akira, S., Takeda, K. & Kaisho, T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2, 675–680 (2001).
Tengvall, S. & Harandi, A.M. Importance of myeloid differentiation factor 88 in innate and acquired immune protection against genital herpes infection in mice. J. Reprod. Immunol. 78, 49–57 (2008).
BenMohamed, L. et al. Induction of CTL response by a minimal epitope vaccine in HLA A*0201/DR1 transgenic mice: dependence on HLA class II restricted T(H) response. Hum. Immunol. 61, 764–779 (2000).
Belyakov, I.M. et al. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. USA 95, 1709–1714 (1998).
Gallichan, W.S. et al. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 166, 3451–3457 (2001).
Kozlowski, P.A. et al. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J. Immunol. 169, 566–574 (2002).
Nesburn, A.B. et al. Functional Foxp3+ CD4+CD25(Bright+) “natural” regulatory T Cells are abundant in rabbit conjunctiva and suppress virus-specific CD4+ and CD8+ effector T cells during ocular herpes infection. J. Virol. 81, 6911–6919 (2007).
Kuklin, N.A., Daheshia, M., Chun, S. & Rouse, B.T. Role of mucosal immunity in herpes simplex virus infection. J. Immunol. 160, 5998–6003 (1998).
Sedlik, C. et al. Intranasal delivery of recombinant parvovirus-like particles elicits cytotoxic T-cell and neutralizing antibody responses. J. Virol. 73, 2739–2744 (1999).
Tengvall, S., Josefsson, A., Holmgren, J. & Harandi, A.M. CpG oligodeoxynucleotide augments HSV-2 glycoprotein D DNA vaccine efficacy to generate T helper 1 response and subsequent protection against primary genital herpes infection in mice. J. Reprod. Immunol. 68, 53–69 (2005).
Eriksson, E.M. & Jackson, D.C. Recent advances with TLR2-targeting lipopeptide-based vaccines. Curr. Protein Pept. Sci. 8, 412–417 (2007).
Nesburn, A.B. et al. Topical/mucosal delivery of sub-unit vaccines that stimulate the ocular mucosal immune system. Ocul. Surf. 4, 178–187 (2006).
Durier, C. et al. Clinical safety of HIV lipopeptides used as vaccines in healthy volunteers and HIV-infected adults. AIDS 20, 1039–1049 (2006).
Moyle, P.M. & Toth, I. Self-adjuvanting lipopeptide vaccines. Curr. Med. Chem. 15, 506–516 (2008).
Borsutzky, S. et al. The mucosal adjuvant macrophage-activating lipopeptide-2 directly stimulates B lymphocytes via the TLR2 without the need of accessory cells. J. Immunol. 174, 6308–6313 (2005).
BenMohamed, L. et al. Identification of novel immunodominant CD4+ Th1-type T-cell peptide epitopes from herpes simplex virus glycoprotein D that confer protective immunity. J. Virol. 77, 9463–9473 (2003).
Carr, D.J. Increased levels of IFN-gamma in the trigeminal ganglion correlate with protection against HSV-1-induced encephalitis following subcutaneous administration with androstenediol. J. Neuroimmunol. 89, 160–167 (1998).
Nesburn, A.B. et al. Local and systemic B cell and Th1 responses induced following ocular mucosal delivery of multiple epitopes of herpes simplex virus type 1 glycoprotein D together with cytosine-phosphate-guanine adjuvant. Vaccine 23, 873–883 (2005).
Kohl, S. et al. Limited antibody-dependent cellular cytotoxicity antibody response induced by a herpes simplex virus type 2 subunit vaccine. J. Infect. Dis. 181, 335–339 (2000).
Gahery-Segard, H. et al. Long-term specific immune responses induced in humans by a human immunodeficiency virus type 1 lipopeptide vaccine: characterization of CD8+-T-cell epitopes recognized. J. Virol. 77, 11220–11231 (2003).
Daubersies, P. et al. Protection against plasmodium falciparum malaria in chimpanzees by immunization with the conserved pre-erythrocytic liver-stage antigen 3. Nat. Med. 6, 1258–1263 (2000).
Milligan, G.N., Bernstein, D.I. & Bourne, N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J. Immunol. 160, 6093–6100 (1998).
Langenberg, A.G. et al. A recombinant glycoprotein vaccine for herpes simplex virus type 2: safety and immunogenicity [corrected]. Ann. Intern. Med. 122, 889–898 (1995).
Sajic, D. et al. Parameters of CpG oligodeoxynucleotide-induced protection against intravaginal HSV-2 challenge. J. Med. Virol. 71, 561–568 (2003).
Pyles, R.B. et al. Use of immunostimulatory sequence-containing oligonucleotides as topical therapy for genital herpes simplex virus type 2 infection. J. Virol. 76, 11387–11396 (2002).
Dumais, N., Patrick, A., Moss, R.B., Davis, H.L. & Rosenthal, K.L. Mucosal immunization with inactivated human immunodeficiency virus plus CpG oligodeoxynucleotides induces genital immune responses and protection against intravaginal challenge. J. Infect. Dis. 186, 1098–1105 (2002).
Daftarian, P. et al. Novel conjugates of epitope fusion peptides with CpG-ODN display enhanced immunogenicity and HIV recognition. Vaccine 23, 3453–3468 (2005).
Davila, E., Kennedy, R. & Celis, E. Generation of antitumor immunity by cytotoxic T lymphocyte epitope peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4 blockade. Cancer Res. 63, 3281–3288 (2003).
BenMohamed, L., Thomas, A. & Druilhe, P. Long-term multiepitopic cytotoxic-T-lymphocyte responses induced in chimpanzees by combinations of Plasmodium falciparum liver-stage peptides and lipopeptides. Infect. Immun. 72, 4376–4384 (2004).
BenMohamed, L. et al. High immunogenicity in chimpanzees of peptides and lipopeptides derived from four new Plasmodium falciparum pre-erythrocytic molecules. Vaccine 18, 2843–2855 (2000).
BenMohamed, L. et al. Lipopeptide immunization without adjuvant induces potent and long-lasting B, T helper, and cytotoxic T lymphocyte responses against a malaria liver stage antigen in mice and chimpanzees. Eur. J. Immunol. 27, 1242–1253 (1997).
Gahery, H. et al. HLA-DR-restricted peptides identified in the Nef protein can induce HIV type 1-specific IL-2/IFN-gamma-secreting CD4+ and CD4+/CD8+ T cells in humans after lipopeptide vaccination. AIDS Res. Hum. Retroviruses 23, 427–437 (2007).
Gahery, H. et al. New CD4+ and CD8+ T cell responses induced in chronically HIV type-1-infected patients after immunizations with an HIV type 1 lipopeptide vaccine. AIDS Res. Hum. Retroviruses 22, 684–694 (2006).
Chentoufi, A.A. et al. Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. J. Virol. 82, 11792–11802 (2008).
Cottalorda, A. et al. TLR2 engagement on CD8 T cells lowers the threshold for optimal antigen-induced T cell activation. Eur. J. Immunol. 36, 1684–1693 (2006).
Zhao, X. et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J. Exp. Med. 197, 153–162 (2003).
Bettahi, I. et al. Protective immunity against ocular herpes infection and disease induced by highly immunogenic self-adjuvanting glycoprotein D lipopeptide vaccines. Invest. Ophthalmol. Vis. Sci. 48, 4643–4653 (2007).
Bettahi, I., Zhang, X., Afifi, R.E. & BenMohamed, L. Protective immunity to genital herpes simplex virus type 1 and type 2 provided by self-adjuvanting lipopeptides that drive dendritic cell maturation and elicit a polarized Th1 immune response. Viral Immunol. 19, 220–236 (2006).
Chentoufi, A.A. et al. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J. Immunol. 180, 426–437 (2008).
Tsunobuchi, H. et al. Memory-type CD8+ T cells protect IL-2 receptor alpha-deficient mice from systemic infection with herpes simplex virus type 2. J. Immunol. 165, 4552–4560 (2000).
Gyotoku, T., Ono, F. & Aurelian, L. Development of HSV-specific CD4+ Th1 responses and CD8+ cytotoxic T lymphocytes with antiviral activity by vaccination with the HSV-2 mutant ICP10DeltaPK. Vaccine 20, 2796–2807 (2002).
Kim, M. et al. Immunodominant epitopes in herpes simplex virus type 2 glycoprotein D are recognized by CD4 lymphocytes from both HSV-1 and HSV-2 seropositive subjects. J. Immunol. 181, 6604–6615 (2008).
Morrison, L.A. Replication-defective virus vaccine-induced protection of mice from genital herpes simplex virus 2 requires CD4 T cells. Virology 376, 205–210 (2008).
Koelle, D.M., Frank, J.M., Johnson, M.L. & Kwok, W.W. Recognition of herpes simplex virus type 2 tegument proteins by CD4 T cells infiltrating human genital herpes lesions. J. Virol. 72, 7476–7483 (1998).
Koelle, D.M. et al. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J. Clin. Invest. 101, 1500–1508 (1998).
Bassett, I. et al. Herpes simplex virus type 2 infection of heterosexual men attending a sexual health centre. Med. J. Aust. 160, 697–700 (1994).
Spruance, S.L. et al. The natural history of recurrent herpes simplex labialis: implications for antiviral therapy. N. Engl. J. Med. 297, 69–75 (1977).
Mikloska, Z. & Cunningham, A.L. Herpes simplex virus type 1 glycoproteins gB, gC and gD are major targets for CD4 T-lymphocyte cytotoxicity in HLA-DR expressing human epidermal keratinocytes. J. Gen. Virol. 79 (Part 2), 353–361 (1998).
Koelle, D.M., Benedetti, J., Langenberg, A. & Corey, L. Asymptomatic reactivation of herpes simplex virus in women after the first episode of genital herpes. Ann. Intern. Med. 116, 433–437 (1992).
Zarling, J.M., Moran, P.A., Brewer, L., Ashley, R. & Corey, L. Herpes simplex virus (HSV)-specific proliferative and cytotoxic T-cell responses in humans immunized with an HSV type 2 glycoprotein subunit vaccine. J. Virol. 62, 4481–4485 (1988).
Zarling, J.M. et al. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. IV. Recognition and activation by cloned glycoproteins gB and gD. J. Immunol. 136, 4669–4673 (1986).
Koelle, D.M. et al. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J. Virol. 68, 2803–2810 (1994).
Koelle, D.M., Gonzalez, J.C. & Johnson, A.S. Homing in on the cellular immune response to HSV-2 in humans. Am. J. Reprod. Immunol. 53, 172–181 (2005).
Koelle, D.M., Schomogyi, M. & Corey, L. Antigen-specific T cells localize to the uterine cervix in women with genital herpes simplex virus type 2 infection. J. Infect. Dis. 182, 662–670 (2000).
Koelle, D.M., Schomogyi, M., McClurkan, C., Reymond, S.N. & Chen, H.B. CD4 T-cell responses to herpes simplex virus type 2 major capsid protein VP5: comparison with responses to tegument and envelope glycoproteins. J. Virol. 74, 11422–11425 (2000).
Khanna, K.M., Lepisto, A.J. & Hendricks, R.L. Immunity to latent viral infection: many skirmishes but few fatalities. Trends Immunol. 25, 230–234 (2004).
van Lint, A. et al. Herpes simplex virus-specific CD8+ T cells can clear established lytic infections from skin and nerves and can partially limit the early spread of virus after cutaneous inoculation. J. Immunol. 172, 392–397 (2004).
Simmons, A. & Tscharke, D.C. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J. Exp. Med. 175, 1337–1344 (1992).
Milligan, G.N., Dudley-McClain, K.L., Young, C.G. & Chu, C.F. T-cell-mediated mechanisms involved in resolution of genital herpes simplex virus type 2 (HSV-2) infection of mice. J. Reprod. Immunol. 61, 115–127 (2004).
Harandi, A.M., Eriksson, K. & Holmgren, J. A protective role of locally administered immunostimulatory CpG oligodeoxynucleotide in a mouse model of genital herpes infection. J. Virol. 77, 953–962 (2003).
Ghiasi, H., Cai, S., Perng, G.C., Nesburn, A.B. & Wechsler, S.L. Both CD4+ and CD8+ T cells are involved in protection against HSV-1 induced corneal scarring. Br. J. Ophthalmol. 84, 408–412 (2000).
Parr, M.B. et al. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab. Invest. 70, 369–380 (1994).
Gillgrass, A.E., Ashkar, A.A., Rosenthal, K.L. & Kaushic, C. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J. Virol. 77, 9845–9851 (2003).
Suvas, S., Kumaraguru, U., Pack, C.D., Lee, S. & Rouse, B.T. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 198, 889–901 (2003).
Acknowledgements
This work was supported by Public Health Service grants EY14900, EY15225 and EY16663, The Discovery Eye Foundation, The Henry L Guenther Foundation, and Research to Prevent Blindness Challenge grant. Dr BenMohamed is an RPB Special Award Investigator. We thank Shizuo Akira for providing TLR2 knockout and MyD88−/− deficient mice, James R. Smiley and Lynda A. Morrison for providing the live attenuated thymidine kinase-deficient HSV-2 (HSV-2 TK−), David M. Koelle for providing the gB vaccinia virus, and Alison Deckhut Augustine and Amy K Stout from the NIH Tetramer Facility for providing the Tetramers used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Chentoufi, A., Dasgupta, G. et al. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol 2, 129–143 (2009). https://doi.org/10.1038/mi.2008.81
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2008.81
This article is cited by
-
Activation of the NLRP3 Inflammasome Is Associated with Valosin-Containing Protein Myopathy
Inflammation (2017)
-
Strategies for intranasal delivery of vaccines
Drug Delivery and Translational Research (2013)
-
Insights into the role of Toll-like receptors in modulation of T cell responses
Cell and Tissue Research (2011)
-
FSL-1, a bacterial-derived toll-like receptor 2/6 agonist, enhances resistance to experimental HSV-2 infection
Virology Journal (2009)