Abstract
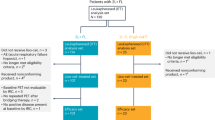
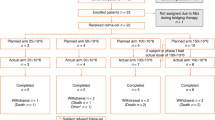
Follicular lymphoma exhibits intratumoral infiltration by non-malignant T lymphocytes, including CD4+CD25+ regulatory T (Treg) cells. We combined denileukin diftitox with rituximab in previously untreated, advanced-stage follicular lymphoma patients anticipating that denileukin diftitox would deplete CD25+ Treg cells while rituximab would deplete malignant B cells. Patients received rituximab 375 mg/m2 weekly for 4 weeks and denileukin diftitox 18 mcg/kg/day for 5 days every 3 weeks for 4 cycles; neither agent was given as maintenance therapy. Between August 2008 and March 2010, 24 patients were enrolled. One patient died before treatment was given and was not included in the analysis. Eleven of 23 patients (48%; 95% confidence interval (CI): 27–69%) responded; 2 (9%) had complete responses and 9 (39%) had partial responses. The progression-free rate at 2 years was 55% (95%CI: 37–82%). Thirteen patients (57%) experienced grade ⩾3 adverse events and one patient (4%) died. In correlative studies, soluble CD25 and the number of CD25+ T cells decreased after treatment; however, there was a compensatory increase in IL-15 and IP-10. We conclude that although the addition of denileukin diftitox to rituximab decreased the number of CD25+ T cells, denileukin diftitox contributed to the toxicity of the combination without an improvement in response rate or time to progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ansell SM, Stenson M, Habermann TM, Jelinek DF, Witzig TE . CD4+ T-cell immune response to large B- cell non-Hodgkin's lymphoma predicts patient outcome. J Clin Oncol 2001; 19: 720–726.
Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM . CD70+ non-Hodgkin lymphoma B cells induce Foxp3 expression and regulatory function in intratumoral CD4+CD25T cells. Blood 2007; 110: 2537–2544.
Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM . Malignant B cells skew the balance of regulatory T cells and TH17 cells in B-cell non-Hodgkin's lymphoma. Cancer Res 2009; 69: 5522–5530.
Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM . Intratumoral CD4+CD25+ regulatory T-cell- mediated suppression of infiltrating CD4+ T-cells in B-cell non-Hodgkin lymphoma. Blood 2006; 107: 3639–3646.
Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM . Attenuation of CD8(+) T-cell function by CD4(+)CD25(+) regulatory T cells in B-cell non-Hodgkin's lymphoma. Cancer Res 2006; 66: 10145–10152.
Shan D, Ledbetter JA, Press OW . Signaling events involved in anti-CD20-induced apoptosis of malignant human B cells. Cancer Immunol Immunother 2000; 48: 673–683.
Golay J, Zaffaroni L, Vaccari T, Lazzari M, Borleri GM, Bernasconi S et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood 2000; 95: 3900–3908.
Wurflein D, Dechant M, Stockmeyer B, Tutt AL, Hu P, Repp R et al. Evaluating antibodies for their capacity to induce cell-mediated lysis of malignant B cells. Cancer Res 1998; 58: 3051–3058.
Hainsworth JD, Litchy S, Burris III HA, Scullin Jr DC, Corso SW, Yardley DA et al. Rituximab as first-line and maintenance therapy for patients with indolent non-hodgkin's lymphoma. J Clin Oncol 2002; 20: 4261–4267.
Hainsworth JD, Litchy S, Shaffer DW, Lackey VL, Grimaldi M, Greco FA . Maximizing therapeutic benefit of rituximab: Maintenance therapy versus re-treatment at progression in patients with indolent non-Hodgkin's lymphoma–a randomized phase II trial of the Minnie Pearl Cancer Res Network. J Clin Oncol 2005; 23: 1088–1095.
Martinelli G, Schmitz SF, Utiger U, Cerny T, Hess U, Bassi S et al. Long-term follow-up of patients with follicular lymphoma receiving single-agent rituximab at two different schedules in trial SAKK 35/98. J Clin Oncol 2010; 28: 4480–4484.
Witzig TE, Vukov AM, Habermann TM, Geyer S, Kurtin PJ, Friedenberg WR et al. Rituximab therapy for patients with newly diagnosed, advanced-stage, follicular grade I non-Hodgkin's lymphoma: a phase II trial in the North Central Cancer Treatment Group. J Clin Oncol. 2005; 23: 1103–1108.
Dang NH, Hagemeister FB, Pro B, McLaughlin P, Romaguera JE, Jones D et al. Phase II study of denileukin diftitox for relapsed/refractory B-Cell non-Hodgkin's lymphoma. J Clin Oncol 2004; 22: 4095–4102.
Dang NH, Fayad L, McLaughlin P, Romaguara JE, Hagemeister F, Goy A et al. Phase II trial of the combination of denileukin diftitox and rituximab for relapsed/refractory B-cell non-Hodgkin lymphoma. Br J Haematol 2007; 138: 502–505.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999; 17: 1244–1253.
Sargent DJ, Chan V, Goldberg RM . A three-outcome design for phase II clinical trials. Control Clin Trials 2001; 22: 117–125.
Kaplan E, Meier P . Nonparametric estimation for incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Solal-Celigny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R et al. Follicular lymphoma international prognostic index. Blood 2004; 104: 1258–1265.
Shevach EM . CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol 2002; 2: 389–400.
Morse MA, Clay TM, Mosca P, Lyerly HK . Immunoregulatory T cells in cancer immunotherapy. Expert Opin Biol Ther 2002; 2: 827–834.
Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 2002; 169: 2756–2761.
Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol 2002; 168: 4272–4276.
Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA . IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J Immunol 2008; 181: 3285–3290.
Sarween N, Chodos A, Raykundalia C, Khan M, Abbas AK, Walker LS . CD4+CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J Immunol 2004; 173: 2942–2951.
Acknowledgements
This work was supported in part by grants CA92104 and CA25224 from the National Institutes of Health and the Predolin Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
SMA received research funding from Eisai Inc. for this study. The remaining authors declare no conflict of interest.
Additional information
Presented in part at the 52nd Annual Meeting of the American Society of Hematology.
Rights and permissions
About this article
Cite this article
Ansell, S., Tang, H., Kurtin, P. et al. Denileukin diftitox in combination with rituximab for previously untreated follicular B-cell non-Hodgkin's lymphoma. Leukemia 26, 1046–1052 (2012). https://doi.org/10.1038/leu.2011.297
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2011.297
Keywords
This article is cited by
-
Novel Therapy Approaches to Follicular Lymphoma
Drugs (2021)
-
The tumour microenvironment in B cell lymphomas
Nature Reviews Cancer (2014)
-
Rational combinations of immunotherapeutics that target discrete pathways
Journal for ImmunoTherapy of Cancer (2013)