Abstract
The purpose of this investigation was to determine whether the high-temperature requirement A-1 (HTRA1) gene polymorphism is associated with age-related macular degeneration (AMD) in native, unrelated Japanese patients. A total of 123 patients with AMD and 133 control subjects without AMD were recruited for this study. The single-nucleotide polymorphism (SNP) rs11200638 in the HTRA1 gene was assessed using a TaqMan assay. The risk A allele frequencies in the AMD cases and control patients were 0.577 and 0.380, respectively, and were associated with a significant risk of developing AMD (p=7.75×10−6). The results were more significant in subtype analyses with wet AMD (p=5.96×10−7). We conclude that the rs11200638 variant in the HTRA1 gene is strongly associated with AMD in the Japanese population. This result supports the hypothesis that the HTRA1 gene may increase susceptibility to AMD development and can participate in a potential new molecular pathway for AMD pathogenesis by extending this association across diverse ethnicities.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in developed countries. There are approximately 8 million people in the United States with features of early or intermediate AMD, of whom approximately 1 million will develop advanced AMD within the next 5 years (Age-Related Eye Disease Study Research Group 2000, 2003, 2005). Currently, AMD is estimated to affect about 50 million people worldwide (Klein et al. 2004). AMD is a clinically heterogeneous and genetically complex disease with multiple genetic and environmental risk factors (Age-Related Eye Disease Study Research Group 2000; Zareparsi et al. 2005). Reported risk factors include ocular pigmentation, dietary factors, positive family history for AMD, high blood pressure, smoking, and several gene mutations, such as ATP binding cassette transporter retina, apolipoprotein E, angiotensin converting enzyme, and fibulin 5 (Age-Related Eye Disease Study Research Group 2000, 2005; Klein et al. 2004; Bok 2005; Allikmets et al. 1997; Allikmets 2000; Klaver et al. 1998; Souied et al. 1998; Hamdi et al. 2002; Stone et al. 2004). Moreover, family-based genome-wide and candidate region linkage studies have successfully identified several major chromosomal regions, including 1q31 and 10q26 (Klein et al. 1998; Weeks et al. 2000; Majewski et al. 2003; Seddon et al. 2003; Kenealy et al. 2004; Abecasis et al. 2004; Fisher et al. 2005).
Recently, the complement factor H (CFH) gene on chromosome 1q31 has been demonstrated as the first major AMD susceptibility gene, and may associate with 30–50% of AMD cases. In the CFH gene, the Y402H variant and other intron variants have been proposed as potentially causative factors in more than ten different Caucasian populations of European descendent (Zareparsi et al. 2005; Klein et al. 2005; Haines et al. 2005; Edwards et al. 2005; Hageman et al. 2005; Li et al. 2006; Maller et al. 2006). Several studies have reported a second major susceptibility genetic locus at chromosome 10q26 for AMD, contributing independently of CFH to disease (Jakobsdottir et al. 2005; Rivera et al. 2005; Schmidt et al. 2006). Very recently, studies of Chinese (DeWan et al. 2006) and Caucasian (Yang et al. 2006) populations have demonstrated the identification of a single-nucleotide polymorphism (SNP) rs11200638 in the promoter region of the high-temperature requirement A-1 (HTRA1) gene polymorphism at this locus.
The purpose of this study is to confirm the association between this novel SNP rs11200638 in the HTRA1 gene and AMD in the Japanese population, as ethnic variation has been reported in AMD-associated Y402H variant and also in other diseases (Okamoto et al. 2006; Gotoh et al. 2006; Grassi et al. 2006; Lau et al. 2006; Uka et al. 2006; Fuse et al. 2006; Chen et al. 2006; Mori et al. 2005). In addition, an important question is whether the HTRA1 variant and smoking are independent risk factors, and investigating this was the second objective of the present study.
Methods
Subjects
The case–control sample was composed of 123 consecutive cases with AMD ranging in age from 51 to 87 years [71.9±8.7; mean±standard deviation (SD)], 89 men and 34 women, and 133 controls without AMD ranging in age from 51 to 88 years (67.9±9.5; mean±SD), 68 men and 65 women, recruited from outpatient visits to the Department of Ophthalmology, Saitama Medical University Hospital in the Saitama prefecture, Japan. All case–control subjects were unrelated, native Japanese Asian. The study was approved by the Ethics Committee of Saitama Medical University, and all procedures were conducted in accordance with the principles of the Declaration of Helsinki. Each individual was fully informed of the purpose of, and the procedures involved in, the study. Informed written consent was obtained for each patient.
Ophthalmic examination, definition, and subtype classification of AMD
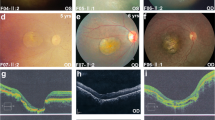
All patients with AMD and the control subjects underwent full ophthalmologic examination, including slit lamp biomicroscopy, funduscopy, and contact lens biomicroscopic examination of the retina. All AMD patients had fluorescein and/or indocyanine green fundus angiography. Complete information regarding diet, family history, systemic conditions, and lifestyle, including smoking, were documented on each subject in a predesigned questionnaire. The visual acuity of AMD patients ranged from hand motion to 20/32. AMD subtypes were diagnosed and classified using the AREDS criteria (Age-Related Eye Disease Study Research Group 2000). The inclusion criteria were as follows: (1) age of 50 years or older, (2) diagnosis of AMD in one or both eyes, (3) no association with other retinochoroidal diseases, such as angioid streaks, high myopia (greater than 6D of myopic refractive error), central serous chorioretinopathy, and presumed ocular histoplasmosis, and (4) positive family history within parents, children, or siblings. There were 104 patients with neovascular (wet form of) AMD and 19 patients with non-neovascular (dry form of) AMD. The control subjects were confirmed not to have clinical evidence of AMD by the same complete ophthalmologic examination that was used to identify the study cohort of AMD patients.
Genotyping and statistical analysis
Genomic DNA was extracted from the peripheral blood of each individual using a DNA extraction and purification kit (Wizard Genomic DNA Purification Kit, Promega, Madison, WI, USA) according to the manufacturer’s instructions. The samples were genotyped using a TaqMan genotyping assay with the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The data were analyzed using the Allelic Discrimination Program (Applied Biosystems).
Genotype and allele frequencies between AMD cases and controls were compared using the Chi-square test for quality of proportions. Hardy-Weinburg equilibrium tests were performed by Chi-square analysis. All analysis was performed using commercially available software (SNPAlyze ver. 6.0, Dynacom, Chiba, Japan).
Results
The distributions of rs11200638 genotype and allele frequencies are given in Table 1. The genotype frequencies in cases and controls were in Hardy-Weinburg equilibrium (p>0.1). The risk A allele frequencies in all AMD cases and control patients were 0.577 and 0.380, respectively, and were associated with a significant risk of developing AMD (χ 2=20.0, p=7.75×10−6). The odds ratio (OR) was 2.23 (95% confidence interval (CI): 1.57–3.18). In comparison to the wild-type homozygous (GG genotype), the ORs for all AMD with the homozygous risk (AA) and heterozygous risk (GA) genotypes were 4.25 (95% CI: 2.13–8.49) and 1.89 (95% CI: 1.04–3.45), respectively. The results were more significant in subtype analyses with wet AMD. The allele frequency Chi-square test yielded a p value of p=5.96×10−7 in comparison between wet AMD cases and control patients (χ 2=24.9). The OR was 2.56 (95% CI: 1.76–3.72). The ORs for wet AMD with AA and GA genotypes were 5.59 (95% CI: 2.66–11.76) and 2.37 (95% CI: 1.22–4.59), respectively, when compared to GG (Table 2).
HTRA1 SNP rs11200638 was also found to have a significant association for AMD in both smokers (subjects who had ever smoked) and nonsmokers (subjects who had never smoked). The association was more significant in nonsmokers than in smokers (p=1.7×10−4 and 1.9×10−2, respectively) (Table 3).
Discussion
In this study, we have demonstrated that the rs11200638 variant in the HTRA1 gene is strongly associated with AMD in the Japanese population. The results were more significant in subtype analyses with wet AMD. The OR for wet AMD associated with the AA and GA genotypes were 5.59 (95% CI: 2.66–11.76) and 2.37 (95% CI: 1.22–4.59), respectively, when compared to the GG genotype. These results are similar to the published data for Chinese (DeWan et al. 2006) and Caucasian (Yang et al. 2006) populations. Replication in diverse ethnic groups worldwide may provide a better appreciation of the role of HTRA1 in AMD pathogenesis. The results presented here support the hypothesis that the HTRA1 gene associates with susceptibility to AMD development, and extends this association across diverse ethnicities. In addition, our data showed that HTRA1 SNP rs11200638 was also found to have a significant association for AMD in smokers and nonsmokers, and the association was more significant in nonsmokers than in smokers. This suggests that HTRA1 plays a role in AMD pathogenesis in both smokers and nonsmokers, and probably more considerably in nonsmokers. Further studies are needed to determine this gene–environment interaction with a larger study population.
The spectrum of clinical presentation or phenotype of Japanese AMD bears some differences compared to that observed in Caucasian AMD. There are also apparent differences in some etiologic factors compared to Western World cultures. In our consecutive case series of patients presenting in an outpatient setting, we had 104 patients with wet AMD, but only 19 patients with dry AMD. These and other epidemiological features characteristic of Asian AMD have been previously reported and include; male predominance, unilateral presentation, a comparatively low incidence of soft drusen, and a greater prevalence of wet AMD (Uyama et al. 1999, 2002; Sho et al. 2003; Bird. 2003; Chang et al. 1999).
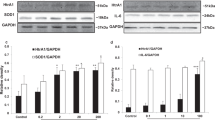
Ethnic variation has been demonstrated in the AMD-associated Y402H variant of the CFH gene. Grassi et al. (2006) have reported the risk C allele frequencies in normal control populations among different ethnicities and they are as follows: Japanese 0.07±0.04, Hispanics 0.17±0.03, African Americans 0.35±0.04, Caucasians 0.34±0.03, and Somalis 0.34±0.03. This result is consistent with the international human haplotype map (HapMap) project database (The International HapMap Consortium 2003). Several Japanese case–control studies have not achieved significance in examining the association of the Y402H variant to AMD (Okamoto et al. 2006; Gotoh et al. 2006; Uka et al. 2006; Fuse et al. 2006). Although there remains a great deal to learn relating to CFH variants in the Chinese population, it appears that they more closely resemble CFH variants in a Japanese population than a Western Caucasian population (Lau et al. 2006; Chen et al. 2006). In contrast to CFH variants, our data demonstrate that the HTRA1 variant in a Japanese population presents similar susceptibility to AMD development with the published findings for the Chinese and Caucasian populations. This finding is also consistent with those of another Japanese study published recently (Yoshida et al. 2007). Yang et al. (2006) have shown that this SNP in the HTRA1 gene is the most likely causal variant for AMD at 10q26 in a Caucasian cohort. They have also found that drusen in the eyes of wet AMD patients were strongly immunolabeled with HTRA1 antibody. DeWan et al. (2006) applied a whole-genome association mapping strategy to a Chinese population and have found a strong association of rs11200638 in the promoter region of the HTRA1 gene and wet AMD. Importantly, this group has demonstrated that rs11200638 is functional in vitro by evaluating ARPE19 and HeLaS3 cells transfected with a relevant luciferase reporter plasmid. They hypothesized that CFH influences the drusen formation characteristic of dry AMD, whereas HTRA1 influences choroidal neovascularization, the hallmark of wet AMD. Magnusson et al. (2006) have demonstrated that the CFH variant confers a similar risk of soft drusen and advanced forms of AMD, and has hypothesized that the CFH variant is a major risk factor for soft drusen formation, but that additional genetic and/or environmental factors may be required for progression to neovascular AMD. The results of our and other studies (Okamoto et al. 2006; Gotoh et al. 2006; Uka et al. 2006; Fuse et al. 2006) in the Japanese population may correlate with Japanese AMD characteristics of a comparatively low incidence of soft drusen and a greater prevalence of wet AMD, and support the hypothesis proposed by Magnusson et al. (2006), DeWan et al. (2006), and Yang et al. (2006).
In summary, this study indicates that the rs11200638 variant in the HTRA1 gene is strongly associated with AMD in an ancestrally and geographically distinct population, as is represented by the Japanese population. This result supports the hypothesis that the HTRA1 gene may increase susceptibility to AMD development and contribute in a potentially novel molecular pathway for AMD pathogenesis.
References
Abecasis GR, Yashar BM, Zhao Y, Ghiasvand NM, Zareparsi S, Branham KE, Reddick AC, Trager EH, Yoshida S, Bahling J, Filippova E, Elner S, Johnson MW, Vine AK, Sieving PA, Jacobson SG, Richards JE, Swaroop A (2004) Age-related macular degeneration: a high-resolution genome scan for susceptibility loci in a population enriched for late-stage disease. Am J Hum Genet 74:482–494
Age-Related Eye Disease Study Research Group (2000) Risk factors associated with age-related macular degeneration. A case–control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology 107:2224–2232
Age-Related Eye Disease Study Research Group (2003) Potential public health impact of Age-Related Eye Disease Study results: AREDS report no. 11. Arch Ophthalmol 121:1621–1624
Age-Related Eye Disease Study Research Group (2005) Risk factors for the incidence of advanced age-related macular degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology 112:533–539
Allikmets R (2000) Further evidence for an association of ABCR alleles with age-related macular degeneration. The International ABCR Screening Consortium. Am J Hum Genet 67:487–491
Allikmets R, Shroyer NF, Sigh N, Seddon JM, Lewis RA, Bernstein PS, Peiffer A, Zabriskie NA, Li Y, Hutchinson A, Dean M, Lupski JR, Leppert M (1997) Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science 277:1805–1807
Bird AC (2003) The Bowman lecture. Towards an understanding of age-related macular disease. Eye 17:457–466
Bok D (2005) Evidence for an inflammatory process in age-related macular degeneration gains new support. Proc Natl Acad Sci USA 102:7053–7054
Chang TS, Hay D, Courtright P (1999) Age-related macular degeneration in Chinese–Canadians. Can J Ophthalmol 34:266–271
Chen LJ, Liu DT, Chan WM, Liu K, Chong KK, Lam DS, Pang CP (2006) Association of complement factor H polymorphisms with exudative age-related macular degeneration. Mol Vis 12:1536–1542
The International HapMap Consortium (2003) The International HapMap Project. Nature 426:789–796
DeWan A, Liu M, Hartman S, Zhang SS, Liu DT, Zhao C, Tam PO, Chan WM, Lam DS, Snyder M, Barnstable C, Pang CP, Hoh J (2006) HTRA1 promoter polymorphism in wet age-related macular degeneration. Science 314:989–992
Edwards AO, Ritter R 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA (2005) Complement factor H polymorphism and age-related macular degeneration. Science 308:421–424
Fisher SA, Abecasis GR, Yashar BM, Zareparsi S, Swaroop A, Iyengar SK, Klein BE, Klein R, Lee KE, Majewski J, Schultz DW, Klein ML, Seddon JM, Santangelo SL, Weeks DE, Conley YP, Mah TS, Schmidt S, Haines JL, Pericak-Vance MA, Gorin MB, Schultz HL, Pardi F, Lewis CM, Weber BH (2005) Meta-analysis of genome scans of age-related macular degeneration. Hum Mol Genet 14:2257–2264
Fuse N, Miyazawa A, Mengkegale M, Yoshida M, Wakusawa R, Abe T, Tamai M (2006) Polymorphisms in Complement Factor H and Hemicentin-1 genes in a Japanese population with dry-type age-related macular degeneration. Am J Ophthalmol 142:1074–1076
Gotoh N, Yamada R, Hiratani H, Renault V, Kuoriwa S, Monet M, Toyoda S, Chida S, Mandai M, Otani A, Yoshimura N, Matsuda F (2006) No association between complement factor H gene polymorphism and exudative age-related macular degeneration in Japanese. Hum Genet 120:139–143
Grassi MA, Fingert JH, Scheetz TE, Roos BR, Ritch R, West SK, Kawase K, Shire AM, Mullins RF, Stone EM (2006) Ethnic variation in AMD-associated complement factor H polymorphism p.Tyr402His. Hum Mutat 27:921–925
Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barrile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R (2005) A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA 102:7227–7232
Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA (2005) Complement factor H variant increases the risk of age-related macular degeneration. Science 308:419–421
Hamdi HK, Reznik J, Castellon R, Atilano SR, Ong JM, Udar N, Tavis JH, Aoki AM, Nesburn AB, Boyer DS, Small KW, Brown DJ, Kenney MC (2002) Alu DNA polymorphism in ACE gene is protective for age-related macular degeneration. Biochem Biophys Res Commun 295:668–672
Jakobsdottir J, Conley YP, Weeks DE, Mah TS, Ferrell RE, Gorin MB (2005) Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet 77:389–407
Kenealy SJ, Schmidt S, Agarwal A, Postel EA, De La Paz MA, Pricak-Vance MA, Haines JL (2004) Linkage analysis for age-related macular degeneration supports a gene on chromosome 10q26. Mol Vis 10:57–61
Klaver CC, Kliffin M, van Duijn CM, Hofman A, Cruts M, Grobbee DE, van Broeckhoven C, de Jong PT (1998) Genetic association of apolipoprotein E with age-related macular degeneration. Am J Hum Genet 63:200–206
Klein ML, Schultz DW, Edwards A, Matise TC, Rust K, Berselli CB, Trzupek K, Welber RG, Ott J, Wirtz MK, Acott TS (1998) Age-related macular degeneration. Clinical features in a large family and linkage to chromosome 1q. Arch Ophthalmol 116:1082–1088
Klein R, Peto T, Bird AC, Vannewkirk MR (2004) The epidemiology of age-related macular degeneration. Am J Ophthlamol 137:486–495
Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J (2005) Complement factor H polymorphism in age-related macular degeneration. Science 308:385–389
Lau LI, Chen SJ, Cheng CY, Yen MY, Lee FL, Lin MW, Hsu WM, Wei YH (2006) Association of the Y402H polymorphism in complement factor H gene and neovascular age-related macular degeneration in Chinese patients. Invest Ophthalmol Vis Sci 47:3242–3246
Li M, Atmaca-Sonmez P, Othman M, Branham KEH, Khanna R, Wade MS, Li Y, Liang L, Zareparsi S, Swaroop A, Abecasis GR (2006) CFH haplotypes without the Y402H coding variant show strong association with susceptibility to age-related macular degeneration. Nat Genet 38:1049–1054
Magnusson KP, Duan S, Sigurdsson H, Petursson H, Yang Z, Zhao Y, Bernstein PS, Ge J, Jonasson F, Stefansson E, Helgadottir G, Zabriskie NA, Jonsson T, Bjomsson A, Thorlacius T, Jonsson PV, Thorleifsson G, Kong A, Stefansson H, Zhang K, Stefansson K, Gulcher JR (2006) CFH Y402H confers similar risk of soft drusen and both forms of advanced AMD. PLoS Med 3:e5
Majewski J, Schultz DW, Weleber RG, Schain MB, Edwards AO, Matise TC, Acott TS, Ott J, Klein ML (2003) Age-related macular degeneration—a genome scan in extended families. Am J Hum Genet 73:540–550
Maller J, George S, Purcell S, Fagerness J, Altshuler D, Daly MJ, Seddon JM (2006) Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Genet 38:1055–1059
Mori M, Yamada R, Kobayashi K, Kawaida R, Yamamoto K (2005) Ethnic differences in allele frequency of autoimmune-disease-associated SNPs. J Hum Genet 50:264–266
Okamoto H, Umeda S, Obazawa M, Minami M, Noda T, Mizota A, Honda M, Tanaka M, Koyama R, Takagi I, Sakamoto Y, Saito Y, Miyake Y, Iwata T (2006) Complement factor H polymorphisms in Japanese population with age-related macular degeneration. Mol Vis 12:156–158
Rivera A, Fisher SA, Fritsche LG, Keilhauer CN, Lichtner P, Meitinger T, Weber BH (2005) Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet 14:3227–3236
Schmidt S, Hauser MA, Scott WK, Postel EA, Agarwal A, Gallins P, Wong F, Chen YS, Spencer K, Schnetz-Boutaud N, Hains JL, Pericak-Vance MA (2006) Cigarette smoking strongly modifies the association of LOC387715 and age-related macular degeneration. Am J Hum Genet 78:852–864
Seddon JM, Santangelo SL, Book K, Chong S, Cote J (2003) A genomewide scan for age-related macular degeneration provides evidence for linkage to several chromosomal regions. Am J Hum Genet 73:780–790
Sho K, Takahashi K, Yamada H, Wada M, Nagai Y, Otsuji T, Nishikawa M, Mitsuma Y, Yamazak Y, Matsumura M, Uyama M (2003) Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol 121:1392–1396
Souied EH, Benlian P, Amouyel P, Feingold J, Lagarde J-P, Munnich A, Kaplan J, Coscas G, Soubrane G (1998) The epsilon4 allele of the apolipoprotein E gene as a potential protective factor for exudative age-related macular degeneration. Am J Ophthalmol 125:353–359
Stone EM, Braun TA, Russell SR, Kuehn MH, Lotery AJ, Moore PA, Eastman CG, Casavant TL, Sheffield VC (2004) Missense variations in the fibulin 5 gene and age-related macular degeneration. N Engl J Med 351:346–353
Uka J, Tamura H, Kobayashi T, Yamane K, Kawakami H, Minamoto A, Mishima HK (2006) No association of complement factor H gene polymorphism and age-related macular degeneration in the Japanese population. Retina 26:985–987
Uyama M, Matsubara T, Fukushima I, Matsunaga H, Iwashita K, Nagai Y, Takahashi K (1999) Idiopathic polypoidal choroidal vasculopathy in Japanese patients. Arch Ophthalmol 117:1035–1042
Uyama M, Wada M, Nagai Y, Matsubara T, Matsunaga H, Fukushima I, Takahashi K, Matsumura M (2002) Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol 133:639–648
Weeks DE, Conley YP, Mah TS, Paul TO, Morse L, Ngo-Chang J, Dailey JP, Ferrell RE, Gorin MB (2000) A full genome scan for age-related maculopathy. Hum Mol Genet 9:1329–1349
Yang Z, Camp NJ, Sun H, Tong Z, Gibbs D, Cameron DJ, Chen H, Zhao Y, Pearson E, Li X, Chien J, DeWan A, Harmon J, Bernstein PS, Shridhar V, Zabriskie NA, Hoh J, Howes K, Zhang K (2006) A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science 314:992–993
Yoshida T, DeWan A, Zhang H, Sakamoto R, Okamoto H, Minami M, Obazawa M, Mizota A, Tanaka M, Saito Y, Takagi I, Hoh J, Iwata T (2007) HTRA1 promoter polymorphism predisposes Japanese to age-related macular degeneration. Mol Vis 13:545–548
Zareparsi S, Branham KEH, Li M, Shah S, Klein RJ, Ott J, Hoh J, Abecasis GR, Swaroop A (2005) Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am J Hum Genet 77:149–153
Acknowledgments
The authors wish to thank Mrs. Hisae Iwata and Mr. Seiji Iwata for their generous gift in support of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Keisuke Mori and Kuniko Horie-Inoue have contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Mori, K., Horie-Inoue, K., Kohda, M. et al. Association of the HTRA1 gene variant with age-related macular degeneration in the Japanese population. J Hum Genet 52, 636–641 (2007). https://doi.org/10.1007/s10038-007-0162-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-007-0162-1
Keywords
This article is cited by
-
Association of the HtrA1 rs11200638 Polymorphism with Neovascular Age-Related Macular Degeneration in Indonesia
Ophthalmology and Therapy (2022)
-
Association of HTRA1 and CFH gene polymorphisms with age-related macular degeneration in Ningbo, China
International Ophthalmology (2021)
-
HTRA1 rs11200638 variant and AMD risk from a comprehensive analysis about 15,316 subjects
BMC Medical Genetics (2020)
-
ARMS2/HTRA1 and CFH polymorphisms are not associated with choroidal neovascularization in highly myopic eyes of the elderly Japanese population
Eye (2010)
-
Association of HTRA1 promoter polymorphism with spinal disc degeneration in Japanese women
Journal of Bone and Mineral Metabolism (2010)