Abstract
We have been performing extensive screening on single nucleotide polymorphisms (SNPs) in and around genes encoding drug metabolizing enzymes, transporters, and receptors and have constructed the high-density SNP maps of such gene regions. In addition to genetic information reported earlier, we identified a total of 390 genetic variations, 358 SNPs and 32 genetic variations of other types, detected in 29 genes encoding G-protein coupled receptors in Japanese populations. Following a comparison of our data with SNPs in the dbSNP database in the US National Center for Biotechnology Information, 156 SNPs from these gene loci are considered to be novel. The fine-scale SNP maps constructed in this study should serve an important resource for studies of linkage-disequilibrium mapping for complex genetic diseases and drug-response phenotypes.
Similar content being viewed by others
Introduction
The G-protein coupled receptors (GPCRs) represent the largest class of cell-surface receptors that recognize extracellular messengers. All members of the GPCR family, which consists of approximately 950 members in humans (Takeda et al. 2002), are predicted to share seven predicted α-helical transmembrane domains, extracellular N-termini and intracellular C-termini, and several conserved structure motifs (Howard et al. 2001). Since these receptors mediate important cellular signals, their mutations and polymorphisms are shown to be responsible for or associated with a large number of diseases. They are also known to be the targets of therapeutic agents; 50% of all modern drugs are considered to target GPCRs (see reviews by George et al. 2002; Spiegel 1995).
To establish “personalized medicine” on the basis of individual genetic variations, we have systematically explored single nucleotide polymorphisms (SNPs) in the genomic regions corresponding to drug-related genes (Iida et al. 2001a–e, 2002a–d, 2004b; Saito et al. 2001a, 2001b, 2002a–d, 2003a; Sekine et al. 2001). As a part of this program, we previously reported SNPs in genomic regions corresponding to genes encoding GPCRs and other known drug targets and constructed fine-scale SNP maps containing more than 1,100 SNPs in 63 genes (Iida et al. 2003, 2004a, 2004b; Saito et al. 2003b). We further extended our SNP discoveries of the GPCRs in 96 chromosomes from healthy Japanese donors and here report a total of 156 novel SNPs and 32 genetic variations of other types for 29 additional members of the GPCR gene family GPR5-9, GPR11-18, GPR20, GPR21-27, GPR29-31, GPR34, GPR35, and GPR37, GPR39, and GPR40.
Subjects and methods
Samples of peripheral blood were obtained with written informed consent form 48 healthy Japanese volunteers. Polymerase chain reaction (PCR) experiments and DNA sequencing were performed according to methods described previously (Iida et al. 2003). In brief, on the basis of genomic sequences corresponding to each of GPCR from the Genbank database in the US National Center for Biotechnology Information (NCBI), we designed primers to amplify all selected genes in their entirety, excluding only regions that corresponded to repetitive sequences as well as intron 1 of GPR39 because it is very long, at 227 kb. Each PCR was performed using 20 ng of a mixture of genomic DNAs from three individuals. All 16 mixed samples were amplified in the GeneAmp PCR system 9700 (PE Applied Biosystems, Foster City, CA, USA) under the following conditions: initial denaturation at 94°C for 2 min followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 30 s, extension at 72°C for 2 min, and post-extension at 72°C for 7 min. Products obtained from the PCR experiments served as templates for direct sequencing and detection of SNPs using the fluorescent dye-terminator cycle-sequencing method. All SNPs detected by the Polyphred computer program (Nickerson et al. 1997) were confirmed by sequencing both strands of each PCR product. All gene names and gene symbols mentioned in this report are according to the nomenclature in LocusLink of the NCBI.
Results and discussion
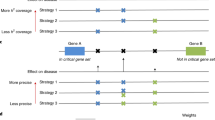
Sequencing of an approximately 183.3-kb genomic region corresponding to the 29 GPCR loci in 96 Japanese chromosomes identified a total of 390 genetic variations, 358 SNPs and 32 genetic variations of other types (Table 1). The overall distribution of SNP was one in every 512 nucleotides on average. By comparing our data with the SNPs deposited in the dbSNP database in the NCBI (as of the end of December 2004), we judged 156 SNPs to be novel (Tables 1, 2). The exon–intron organization of each gene and locations of SNPs identified within each locus are shown schematically in Fig.1; detailed information is given in Table 2. Sub-regional distributions of novel SNPs were as follows: 44 in 5′ flanking regions, five in 5′ untranslated regions (UTRs), 11 in coding regions, 47 in introns, 12 in 3′ UTRs, and 37 in 3′ flanking regions. The overall frequencies of nucleotide substitutions were counted as 28% for A/G, 28% for C/T, 17% for A/C, 14% for C/G, 9% for G/T, and 4% for A/T. The transitions occurred 1.3 times more frequently than transversions. In addition, of the 19 SNPs in coding regions, we found a total of eight novel nonsynonymous substitutions: 404A>T (Tyr135Phe) in exon 1 of GPR7, 217C>G (Leu73Val) in exon 2 of GPR11, 149C>T (Ala50Val) in exon 1 and 371G>A (Arg124His) in exon 1 of GPR14, 235G>T (Ala79Ser) in exon 2 of GPR16, 343G>A (Val115Ile) in exon 1 of GPR21, 1201G>A (Gly401Arg) in exon 2 of GPR24, and 743G>A (Arg248His) in exon 2 of GPR30. These SNPs might effect on the function of the corresponding GPCRs.
Fine-scale single nucleotide polymorphism (SNP) maps of 29 gene loci encoding G-protein coupled receptors (GPCRs). Exons and introns are represented by rectangles and horizontal lines, respectively. The SNPs are indicated above the lines (designations correspond to the left-most column of Table 2). Genetic variations of other types, where present, are indicated below the maps. However, the complete 5′ untranslated sequences and/or 3′ untranslated sequences of GPR5, GPR6, GPR7, GPR8, GPR14, GPR20, GPR21, GPR22, GPR25, GPR27, GPR31, GPR35, GPR39 and GPR40 were yet unidentified in database we used
Altogether, we have collected a total of 156 novel SNPs and 32 genetic variations of other types by screening of 29 genes encoding GPCRs. We hope our SNP catalog can contribute to further investigations for identifying genes associated with drug efficacy and/or adverse drug reactions and for designing personalized medical care.
References
den Dunnen JT, Antonarakis SE (2000) Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat 15:7–12
George SR, O’Dowd BF, Lee SP (2002) G-protein coupled receptor oligomerization and its potential for drug discovery. Nat Rev Drug Discov 1:808–820
Howard AD, McAllister G, Feighner SD, Liu Q, Nargund RP, Van der Ploeg LH, Patchett AA (2001) Orphan G-protein coupled receptors and natural ligand discovery. Trends Pharmacol Sci 22:132–140
Iida A, Sekine A, Saito S, Kitamura Y, Kitamoto T, Osawa S, Mishima C, Nakamura Y (2001a) Catalog of 320 single nucleotide polymorphisms (SNPs) in 20 quinone oxidoreductase and sulfotransferase genes. J Hum Genet 46:225–240
Iida A, Saito S, Sekine A, Kitamoto T, Kitamura Y, Mishima C, Osawa S, Kondo K, Harigae S, Nakamura Y (2001b) Catalog of 434 single-nucleotide polymorphisms (SNPs) in genes of the alcohol dehydrogenase, glutathione S-transferase, and nicotinamide adenine dinucleotide, reduced (NADH) ubiquinone oxidoreductase families. J Hum Genet 46:385–407
Iida A, Saito S, Sekine A, Kitamura Y, Kondo K, Mishima C, Osawa S, Harigae S, Nakamura Y (2001c) High-density single-nucleotide polymorphism (SNP) map of the 150-kb region corresponding to the human ATP-binding cassette transporter A1 (ABCA1) gene. J Hum Genet 46:522–528
Iida A, Saito S, Sekine A, Harigae S, Osawa S, Mishima C, Kondo K, Kitamura Y, Nakamura Y (2001d) Catalog of 46 single-nucleotide polymorphisms (SNPs) in the microsomal glutathione S-transferase 1 (MGST1) gene. J Hum Genet 46:590–594
Iida A, Saito S, Sekine A, Mishima C, Kondo K, Kitamura Y, Harigae S, Osawa S, Nakamura Y (2001e) Catalog of 258 single-nucleotide polymorphisms (SNPs) in genes encoding three organic anion transporters, three organic anion-transporting polypeptides, and three NADH:ubiquinone oxidoreductase flavoproteins. J Hum Genet 46:668–683
Iida A, Saito S, Sekine A, Kondo K, Mishima C, Kitamura Y, Harigae S, Osawa S, Nakamura Y (2002b) Thirteen single-nucleotide polymorphisms (SNPs) in the alcohol dehydrogenase 4 (ADH4) gene locus. J Hum Genet 47:74–76
Iida A, Saito S, Sekine A, Mishima C, Kitamura Y, Kondo K, Harigae S, Osawa S, Nakamura Y (2002c) Catalog of 605 single-nucleotide polymorphisms (SNPs) among 13 genes encoding human ATP-binding cassette transporters: ABCA4, ABCA7, ABCA8, ABCD1, ABCD3, ABCD4, ABCE1, ABCF1, ABCG1, ABCG2, ABCG4, ABCG5, and ABCG8. J Hum Genet 47:285–310
Iida A, Saito S, Sekine A, Mishima C, Kitamura Y, Kondo K, Harigae S, Osawa S, Nakamura Y (2002d) Catalog of 86 single-nucleotide polymorphisms (SNPs) in three uridine diphosphate glycosyltransferase genes: UGT2A1, UGT2B15, and UGT8. J Hum Genet 47:505–510
Iida A, Saito S, Sekine A, Mishima C, Kitamura Y, Kondo K, Harigae S, Osawa S, Nakamura Y (2002a) Catalog of 77 single-nucleotide polymorphisms (SNPs) in the carbohydrate sulfotransferase 1 (CHST1) and carbohydrate sulfotransferase 3 (CHST3) genes. J Hum Genet 47:14–19
Iida A, Saito S, Sekine A, Mishima C, Kitamura Y, Kondo K, Harigae S, Osawa S, Nakamura Y (2003) Catalog of 668 SNPs detected among 31 genes encoding potential drug targets on the cell surface. J Hum Genet 48:23–46
Iida A, Saito S, Sekine A, Kataoka Y, Tabei W, Nakamura Y (2004a) Catalog of 300 SNPs in 23 genes encoding G-protein coupled receptors. J Hum Genet 49:194–208
Iida A, Saito S, Sekine A, Tabei W, Kataoka Y, Nakamura Y (2004b) Identification of 20 novel SNPs in the guanine nucleotide binding protein alpha 12 gene locus. J Hum Genet 49:445–448
Nickerson DA, Tobe VO, Taylor SL (1997) PolyPhred: automating the detection and genotyping of single nucleotide substitutions using fluorescence-based resequencing. Nucleic Acids Res 25:2745–2751
Saito S, Iida A, Sekine A, Eguchi C, Miura Y, Nakamura Y (2001a) Seventy genetic variations in human microsomal and soluble epoxide hydrolase genes (EPHX1 and EPHX2) in the Japanese population. J Hum Genet 46:325–329
Saito S, Iida A, Sekine A, Miura Y, Sakamoto T, Ogawa C, Kawauchi S, Higuchi S, Nakamura Y (2001b) Identification of 197 genetic variations in six human methyltranferase genes in the Japanese population. J Hum Genet 46:529–537
Saito S, Iida A, Sekine A, Miura Y, Ogawa C, Kawauchi S, Higuchi S, Nakamura Y (2002a) Three hundred twenty-six genetic variations in genes encoding nine members of ATP-binding cassette, subfamily B (ABCB/MDR/TAP), in the Japanese population. J Hum Genet 47:38–50
Saito S, Iida A, Sekine A, Miura Y, Ogawa C, Kawauchi S, Higuchi S, Nakamura Y (2002b) Identification of 779 genetic variations in eight genes encoding members of the ATP-binding cassette, subfamily C (ABCC/MRP/CFTR). J Hum Genet 47:147–171
Saito S, Iida A, Sekine A, Ogawa C, Kawauchi S, Higuchi S, Ohno M, Nakamura Y (2002c) 906 variations among 27 genes encoding cytochrome P450 (CYP) enzymes and aldehyde dehydrogenases (ALDHs) in the Japanese population. J Hum Genet 47:419–444
Saito S, Iida A, Sekine A, Ogawa C, Kawauchi S, Higuchi S, Nakamura Y (2002d) Catalog of 238 variations among six human genes encoding solute carriers (hSLCs) in the Japanese population. J Hum Genet 47:576–584
Saito S, Iida A, Sekine A, Kawauchi S, Higuchi S, Ogawa C, Nakamura Y (2003a) Catalog of 680 variations among eight cytochrome p450 (CYP) genes, nine esterase genes, and two other genes in the Japanese population. J Hum Genet 48:249–270
Saito S, Iida A, Sekine A, Kawauchi S, Higuchi S, Ogawa C, Nakamura Y (2003b) Catalog of 178 variations in the Japanese population among eight human genes encoding G protein-coupled receptors (GPCRs). J Hum Genet 48:461–468
Sekine A, Saito S, Iida A, Mitsunobu Y, Higuchi S, Harigae S, Nakamura Y (2001) Identification of single-nucleotide polymorphisms (SNPs) of human N-acetyltransferase genes NAT1, NAT2, AANAT, ARD1 and L1CAM in the Japanese population. J Hum Genet 46:314–319
Spiegel AM (1995) Defects in G protein-coupled signal transduction in human disease. Annu Rev Physiol 58:143–170
Takeda S, Kadowaki S, Haga T, Takaesu H, Mitaku S (2002) Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett 520:97–101
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iida, A., Nakamura, Y. Identification of 156 novel SNPs in 29 genes encoding G-protein coupled receptors. J Hum Genet 50, 182–191 (2005). https://doi.org/10.1007/s10038-005-0238-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-005-0238-8