Abstract
An in vivo-mimic silkworm infection model with Mycobacterium smegmatis was established. When silkworms were raised at 37 °C following an injection of M. smegmatis cells (1.25 × 107 CFU larva−1 g−1) into the silkworm hemolymph, they died within 48 h. Under these conditions, four microbial peptides with anti-M. smegmatis activity, lariatin A, calpinactam, lysocin E and propeptin, exerted therapeutic effects in a dose-dependent manner, and these are also clinically used agents that are active against Mycobacterium tuberculosis. These results indicate that the silkworm infection model with M. smegmatis is practically useful for the screening of therapeutically effective anti-M. tuberculosis antibiotics.
Similar content being viewed by others
Introduction
In the process of antibiotic discovery, candidate compounds active against pathogenic microorganisms in an in vitro assay system often have no therapeutic effects in in vivo animal infection models. In order to overcome this issue, the therapeutic efficacies of candidate compounds need to be evaluated in an in vivo-mimic assay at the early stage of drug development. In our previous studies, a silkworm infection model with methicillin-resistant Staphylococcus aureus (MRSA) was established for the primary screening of anti-MRSA antibiotics, and led to the discovery of the new antibiotics, nosokomycins and lysocins.1, 2, 3, 4 Nosokomycin A and lysocin E exerted therapeutic effects in a mouse infection model with MRSA.1 This silkworm infection was found to be applicable to other pathogenic microorganisms such as Pseudomonas aeruginosa and Candida albicans.5, 6, 7, 8 The silkworm infection model has the following advantages over the mouse infection model: fewer ethical issues, lower maintenance costs, less space required to keep animals, less drugs required for evaluations and shorter times for infection experiments.6, 7
Tuberculosis (TB), caused by Mycobacterium tuberculosis, is one of the major infectious diseases and is still the greatest single infectious cause of mortality worldwide.9 However, anti-TB agents in clinical use are more limited than agents available for other bacterial infections.10 Therefore, new anti-TB agents with different mechanisms of action are desired. We have screened for anti-TB antibiotics using Mycobacterium smegmatis instead of M. tuberculosis because M. smegmatis is nonpathogenic (utilized in a normal-level laboratory), grows faster than other Mycobacterium spp. and is sensitive to clinically used anti-TB agents. As a result, lariatins (lasso peptides, Figure 1) and calpinactam (a hexapeptide, Figure 1) were discovered from microorganisms in this assay, and were found to exhibit anti-TB activities in vitro.11, 12, 13, 14 As the next step, the in vivo efficacies of these microbial peptides need to be tested.
In the present study, an in vivo-mimic silkworm infection model with M. smegmatis was established and anti-mycobacterial compounds including clinically used anti-TB drugs and microbial peptides were evaluated using this silkworm infection assay.
Materials and Methods
Materials
Lariatin A,11, 12 lysocin E1 and propeptins15, 16 were purified from a culture broth of R. jostii K01-B0171, Lysobacter sp. RH2180-5 and Microbispora sp. SNA-115, respectively. Calpinactam was synthesized as reported previously.17 Rifampicin (RFP), streptomycin (SM) and kanamycin (KM) were purchased from Wako Pure Chemical Industries (Osaka, Japan), isoniazid (INH) was purchased from Sigma Aldrich (St Louis, MO, USA), ethambutol (EB) was purchased from LKT Laboratories (St Paul, MN, USA) and pyrazinamide (PZA) was obtained from Tokyo Chemical Industries (Tokyo, Japan). Unless otherwise stated, all other reagents were reagent-grade commercial products. Middlebrook 7H9 broth (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) containing 0.5% Tween-80 (Tokyo Chemical Industries) and 10% albumin dextrose catalase (ADC) enrichment (5% bovine serum albumin, Sigma Aldrich; 2% glucose, Wako Pure Chemical Industries; 0.85% NaCl, Wako Pure Chemical Industries) were used for the cultivation of M. smegmatis M341.
Fertilized silkworm eggs, Bombyx mori (Hu•Yo × Tukuba•Ne), were purchased from Ehime Sansyu (Ehime, Japan) and fed artificial food (Silk Mate 2S, Nihon Nosan Kogyo, Kanagawa, Japan; and Silkmate, Katakura Industries, Tokyo, Japan) until the fourth-instar larvae stage.
Preparation of M. smegmatis suspension
M. smegmatis M341 was stored in 20% glycerol at −80 °C. The frozen stock culture was scraped with a sterile loop, inoculated in Middlebrook 7H9 broth (5 ml) in a T-25 flask (Corning, Corning, NY, USA) and cultured under static conditions at 37 °C for 48 h (∼5.0 × 108 CFU ml−1).
MIC values using the liquid microdilution method
The MIC values of the anti-TB antibiotics (INH, RFP, PZA, EB, SM and KM) and microbial peptides (lariatin A, calpinactam, lysocin E, propeptin and propeptin-2) against M. smegmatis were obtained using the liquid microdilution method.12 An M. smegmatis M341 suspension was adjusted to 1.0 × 106 CFU ml−1 in Middlebrook 7H9 broth containing 0.5% Tween-80 and 10% ADC enrichment. The suspension (95 μl) was added to each well of a 96-well microplate (Corning) with or without the test drugs (5 μl in MeOH or water) and incubated at 37 °C for 30 h. MTT reagent (5.5 mg ml−1 Thiazolyl Blue Tetrazolium Bromide, Sigma Aldrich; 5 μl) was added to each well and the cells were incubated for 16 h. After cells had been lysed with lysis buffer (40% N,N-dimethylformamide, Nacalai Tesque, Kyoto, Japan; 20% SDS, Wako Pure Chemical Industries; 2% acetic acid, Kanto Chemical, Tokyo, Japan; 95 μl), the absorbance of the lysate was measured at 570 nm using an absorption spectrometer (Power Wave X340, Bio Tek Instruments, Winooski, VT, USA). The MIC value was defined as the lowest drug concentration that showed 90% growth inhibition of M. smegmatis.
Silkworm infection assay with M. smegmatis
Hatched silkworm larvae were raised by feeding an artificial diet containing antibiotics (Silk Mate 2S, Nihon Nosan Kogyo) in an incubator at 27 °C until the fourth molting stage. On the first day of fifth-instar larvae, silkworms were fed an antibiotic-free artificial diet (Silkmate, Katakura Industries) for 24 h. On the second day, a twofold serially diluted M. smegmatis M341 suspension (0.6 × 107 to 2.5 × 107 CFU larva−1 g−1 in 50 μl Middlebrook 7H9 broth) was injected into the hemolymph through the dorsal surface of the silkworms (2.0 g, n=5) using a disposable 1 ml syringe with a 27-G needle (TERUMO, Tokyo, Japan). The silkworms were incubated without feed at 37 or 27 °C unless stated otherwise, and their survival rate was measured every 2 h after drug injection.
ED50 values in the silkworm infection assay with M. smegmatis
An M. smegmatis M341 suspension (1.25 × 107 CFU larva−1 g−1 in 50 μl Middlebrook 7H9 broth) was injected into the hemolymph of silkworm larvae (2.0 g, n=5), followed by the injection of anti-TB-antibiotics or microbial peptides (50 μl in water or 10% DMSO) within 30 min. Silkworms were maintained at 37 °C. The survival rate of the indicated drug dose was assessed 48 h after drug injection. ED50 values were defined as the amount of a drug required for a 50% survival rate, normalized per 1 g of silkworm.
Results
Establishment of a silkworm infection model with M. smegmatis
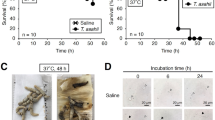
M. smegmatis is a nonpathogenic bacterium. In order to establish a silkworm infection assay with M. smegmatis, the temperature (27 and 37 °C) for silkworm breeding and colony number of the bacterium (0.6 × 107 to 2.5 × 107 CFU larva−1 g−1) injected into silkworms were investigated. As shown in Figure 2, when silkworms injected with M. smegmatis (2.5 × 107 CFU larva−1 g−1) were incubated at 37 °C, all (n=5) died within 42 h. In contrast, an incubation at 27 °C allowed all silkworms to survive, even after 48 h. Therefore, the incubation temperature for infected silkworms was set at 37 °C. Three different cell numbers (0.6 × 107 to 2.5 × 107 CFU larva−1 g−1) of M. smegmatis were subsequently injected into silkworms, and infected silkworms were incubated for 60 h. As shown in Figure 2, silkworms started to die after 36 to 48 h in a cell number-dependent manner. Injections of 0.6 × 107, 1.3 × 107 and 2.5 × 107 CFU larva−1 g−1 caused all silkworms to die within 60, 44 and 40 h, respectively. Therefore, the cell number of M. smegmatis injected into silkworms was set at 1.25 × 107 CFU larva−1 g−1. Furthermore, the supernatant or an autoclaved suspension of M. smegmatis showed no pathogenicity (data not shown). Based on these results, the conditions for the silkworm infection model with M. smegmatis were established; the bacterium injected at 1.25 × 107 CFU larva−1 g−1 and infected silkworms incubated at 37 °C. Under these conditions, all silkworms die within 48 h.
Silkworm killing ability of Mycobacterium smegmatis. A suspension of the M. smegmatis M341 strain was diluted to the indicated cell number and injected into the silkworm hemolymph. Infected silkworms were incubated at 37 °C. The number of surviving silkworms was counted 60 h after the injection. ♦: 2.5 × 107;  : 1.3 × 107;
: 1.3 × 107;  : 0.6 × 107; and
: 0.6 × 107; and  : 0 CFU larva−1 g−1. Experiments were performed three times and reproducible data were observed.
: 0 CFU larva−1 g−1. Experiments were performed three times and reproducible data were observed.
In vitro anti-M. smegmatis activity of microbial peptides
The in vitro anti-M. smegmatis activities of clinically used anti-TB agents (INH, RFP, PZA, EB, SM and KM) and microbial peptides (lariatin A, calpinactam, lysocin E, propeptin and propeptin-2, in Figure 1) were compared under the same conditions using the liquid microdilution method.12 The MIC values are summarized in Table 1. INH, RFP, EB, SM and KM exhibited anti-M. smegmatis activities with MIC values of 1.56, 1.56, 0.78, 0.78 and 3.13 μg ml−1, respectively. However, pyrazinamide showed no anti-M. smegmatis activity, even at 100 μg ml−1. These results are consistent with previous findings.18
The MIC values of the five microbial peptides against M. smegmatis are also shown in Table 1. Lariatin A, calpinactam, lysocin E and propeptin exhibited anti-M. smegmatis activity with MIC values of 0.10, 0.78, 3.13 and 100 μg ml−1, respectively. However, propeptin-2, an inactive form of propeptin,16 showed no anti-mycobacterial activity, even at 100 μg ml−1. We confirmed the anti-M. smegmatis activities of lariatin A, calpinactam and propeptin. Lysocin E exhibited anti-M. smegmatis activity for the first time. The potency of activity was in the order of lariatin A>lysocin E>calpinactam>>propeptin.
Therapeutic efficacies of microbial peptides in the silkworm infection assay with M. smegmatis
Anti-TB agents were evaluated in the silkworm infection assay (n=5) with M. smegmatis. When INH, RFP, EB, SM and KM were injected, infected silkworms survived in a dose-dependent manner (Table 1 and Figure 3). However, PZA did not exert any therapeutic effects, even at 50 μg larva−1 g−1 (Table 1). Furthermore, none of the anti-TB antibiotics (50 μg larva−1 g−1) exhibited toxicity against silkworms, at least for 48 h (data not shown). The ED50 values of anti-TB drugs against M. smegmatis are summarized in Table 1. Among them, the two aminoglycoside antibiotics (KM and SM) exerted strong therapeutic effects in the silkworm assay.
Therapeutic effects of anti-tuberculosis (TB) drugs in the silkworm infection assay with Mycobacterium smegmatis. (a) Isoniazid (INH), (b) rifampicin (RFP), (c) pyrazinamide (PZA), (d) ethambutol (EB), (e) streptomycin (SM), and (f) kanamycin (KM). ♦: 50;  : 25;
: 25;  : 13;
: 13;  : 5; □: 1.5; ○: 0.5; and
: 5; □: 1.5; ○: 0.5; and  : 0 μg larva−1 g−1. Experiments were performed three times and reproducible data were observed.
: 0 μg larva−1 g−1. Experiments were performed three times and reproducible data were observed.
The five microbial peptides were evaluated in the silkworm infection assay with M. smegmatis (n=5). As shown in Figure 2, when lariatin A, calpinactam, lysocin E and propeptin were injected, silkworms survived in a dose-dependent manner. The ED50 values of the peptides against M. smegmatis are summarized in Table 1. Lariatin A and lysocin E exerted strong therapeutic effects with the same ED50 value of 0.5 μg larva−1 g−1. Propeptin displayed very weak therapeutic effects, whereas propeptin-2 showed no effects, even at 50 μg larva−1 g−1 (Figure 4). None of the peptides (50 μg larva−1 g−1) exhibited toxicity against silkworms, at least for 48 h (data not shown).
Therapeutic effects of microbial peptides in the silkworm infection assay with Mycobacterium smegmatis. (a) Lariatin A, (b) calpinactam, (c) lysocin E, (d) propeptin and (e) propeptin-2. ♦: 50;  : 25;
: 25;  : 13;
: 13;  : 5; □: 1.5; ○: 0.5; and
: 5; □: 1.5; ○: 0.5; and  : 0 μg larva−1 g−1. Experiments were performed three times and reproducible data were observed.
: 0 μg larva−1 g−1. Experiments were performed three times and reproducible data were observed.
Discussion
In the present study, an in vivo-mimic silkworm infection model with M. smegmatis was established. Although the incubation temperature is typically 27 °C for silkworms infected with most pathogenic microorganisms, a higher temperature of 37 °C is needed for M. smegmatis-infected silkworms to die within 60 h. Furthermore, a higher cell number (107 level CFU per larva for M. smegmatis vs 106 level CFU per larva for other pathogenic microorganisms) is required for injection in this silkworm assay. As mycobacteria are very slow-growing bacteria, these two conditions are important for this assay to be reproducible and work well. It is important to note that the 37 °C incubation time was limited to within 60 h, even for control silkworms (Figure 2).
Clinically used anti-TB drugs were evaluated under these established conditions (Figure 3). The potency of in vitro anti-M. smegmatis activity (MIC in Table 1) was in the order of EB=SM>INH=RFP>KM>>PZA, whereas that in the silkworm assay (ED50 in Table 1) was KM>SM>RFP>INH>EB>>PZA. PZA did not exhibit any activity in the in vitro or silkworm assay. According to its mechanism of action on mycobacteria, PZA is converted to the active metabolite pyrazinoic acid by the enzyme pyrazinamidease in M. tuberculosis.19 M. smegmatis also exhibits pyrazinamidease activity, but is resistant to PZA because PZA cannot enter the cells.19 Therefore, anti-TB PZA exerts no effects on M. smegmatis in either assay. EB showed very potent anti-M. smegmatis and anti-TB activities in vitro; however, EB was required at the highest dose to exhibit therapeutic efficacy in M. tuberculosis-infected mice20 and M. smegmatis-infected silkworms. INH is a prodrug that needs to be activated by KatG, an enzyme with the dual activities of catalase and peroxidase, in order to show its anti-mycobacterial activity.21 As INH displayed good efficacy in the silkworm assay, the activation reaction may occur in infected silkworms. Thus, the efficacies of the anti-TB agents in the silkworm infection assay with M. smegmatis were found to be consistent with those in the mouse infection assay with M. tuberculosis.20 We consider this silkworm infection model with M. smegmatis to be applicable to evaluations of the in vivo effectiveness of candidate compounds as anti-TB agents.
Four microbial peptides reported to exhibit in vitro anti-mycobacterial/anti-Gram-positive bacterial activities were then evaluated in this silkworm assay. Lariatin A produced by Rhodococcus jostii K01-B0171 and calpinactam produced by Mortierella alpina FKI-4905 were discovered in the screening for antibiotics selectively active against M. smegmatis, and have also been reported to show in vitro anti-TB activities.12, 13 As shown in Figure 1, lariatin A forms a unique lasso peptide,11 whereas calpinactam is a hexapeptide with a caprolactam ring at its C terminus.14 Lysocin E produced by Lysobacter sp. RH2180-5 was discovered in the in vitro anti-S. aureus assay and its therapeutic effects were confirmed in the silkworm infection assay with S. aureus.1 Lysocin E (Figure 1) is a cyclic depsipeptide that is active against Gram-positive bacteria by targeting the bacterial membrane menaquinone. In the present study, lysocin E exhibited potent anti-M. smegmatis activity in vitro (MIC, 0.10 μg ml−1) for the first time (Table 1). Propeptin and propeptin-2 produced by Microbispora sp. SNA-115 were discovered in the course of screening for prolyl endopeptidase inhibitors.15, 16 Propeptins appear to be lasso-type peptides (Figure 1); however, direct evidence to support this has not yet been obtained. Propeptin was also reported to exhibit moderate anti-Mycobacterium phlei activity in vitro, whereas propeptin-2 lost its anti-Mycobacterium activity.16 Corresponding in vitro results for the two propeptins against M. smegmatis were observed (Table 1). As shown in Figure 3, lariatin A, calpinactam, lysocin E and propeptin exerted therapeutic effects in a dose-dependent manner in the silkworm infection assay, whereas propeptin-2 did not, even at the highest dose. Lysocin E and lariatin A were particularly potent, with the same ED50 values of 0.50 μg larva−1 g−1 (Table 1). The therapeutic effects of the microbial peptides in this silkworm infection assay correlated with potencies in the in vitro anti-M. smegmatis assay (Table 1).
Hamamoto et al.7 previously reported that the ratio of ED50 per MIC of a compound is an index of drug potential, and the ratio is typically <10 for clinically used antibiotics. As shown in Table 1, INH, RFP, SM and KM had ratios of <10, whereas that of EB was 64. The ratios of lariatin A, calpinactam, lysocin E and propeptin were <10 (Table 1), indicating the potential of these peptides as anti-TB drugs.
In conclusion, we established a silkworm infection model with M. smegmatis as an in vivo-mimic evaluation for anti-TB agent development, and demonstrated the therapeutic efficacies of clinically used anti-TB drugs and microbial peptides in this infection assay. The silkworm infection model will be utilized as a practically effective assay to develop anti-TB drugs. We identified four microbial peptides as potential anti-TB drug candidates.
References
Hamamoto, H. et al. Lysocin E is a new antibiotic that targets menaquinone in the bacterial membrane. Nat. Chem. Biol. 11, 127–133 (2015).
Uchida, R. et al. Nosokomycins, new antibiotics, discovered in an in vivo-mimic infection model using silkworm larvae. I. Fermentation, isolation and biological properties. J. Antibiot. 63, 151–155 (2010).
Uchida, R., Iwatsuki, M., Kim, Y. P., Ōmura, S. & Tomoda, H. Nosokomycins, new antibiotics, discovered in an in vivo-mimic infection model using silkworm larvae. II. Structure elucidation. J. Antibiot. 63, 157–163 (2010).
Uchida, R. et al. In vitro and in vivo anti-MRSA activities of nosokomycins. Drug. Discov. Ther. 8, 249–254 (2014).
Uchida, R., Namiguchi, S., Ishijima, H. & Tomoda, H. Therapeutic effects of three trichothecenes in the silkworm infection assay with Candida albicans. Drug. Discov. Ther. 10, 44–48 (2016).
Kaito, C., Akimitsu, N., Watanabe, H. & Sekimizu, K. Silkworm larvae as an animal model of bacterial infection pathogenic to humans. Microb. Pathog. 32, 183–190 (2002).
Hamamoto, H. et al. Quantitative evaluation of the therapeutic effects of antibiotics using silkworms infected with human pathogenic microorganisms. Antimicrob. Agents Chemother. 48, 774–779 (2004).
Matsumoto, Y. et al. Quantitative evaluation of cryptococcal pathogenesis and antifungal drugs using a silkworm infection model with Cryptococcus neoformans. J. Appl. Microbiol. 112 (1), 138–146 (2012).
National Institute of Allergy and Infectious Diseases (NIAID). https://www.niaid.nih.gov/diseases-conditions/tuberculosis-tb. Accessed on 10 February 2016.
World Health Organization. Treatment of tuberculosis: guidelines. 4th edn http://apps.who.int/iris/bitstream/10665/44165/1/9789241547833_eng.pdf?ua=1&ua=1. Accessed on 10 February (2016).
Iwatsuki, M. et al. Lariatins, antimycobacterial peptides produced by Rhodococcus sp. K01-B0171, have a lasso structure. J. Am. Chem. Soc. 128, 7486–7491 (2006).
Iwatsuki, M. et al. Lariatins, novel anti-mycobacterial peptides with a lasso structure, produced by Rhodococcus jostii K01-B0171. J. Antibiot. 60, 357–363 (2007).
Koyama, N. et al. Calpinactam, a new anti-mycobacterial agent, produced by Mortierella alpina FKI-4905. J. Antibiot. 63, 183–186 (2010).
Koyama, N. et al. Structure and total synthesis of fungal calpinactam, a new antimycobacterial agent. Org. Lett. 12, 432–435 (2010).
Kimura, K. et al. Propeptin, a new inhibitor of prolyl endopeptidase produced by Microbispora. I. Fermentation, isolation and biological properties. J. Antibiot. 50, 373–378 (1997).
Kimura, K. et al. Novel propeptin analog, propeptin-2, missing two amino acid residues from the propeptin C-terminus loses antibiotic potency. J. Antibiot. 60, 519–523 (2007).
Nagai, K., Koyama, N., Sato, N., Yanagisawa, C. & Tomoda, H. Synthesis and antimycobacterial activity of calpinactam derivatives. Bioorg. Med. Chem. Lett. 22, 7739–7741 (2012).
Lee, J. et al. The sensititre MYCOTB MIC plate for testing Mycobacterium tuberculosis susceptibility to first- and second-line drugs. Antimicrob. Agents. Chemother. 58, 11–18 (2014).
Raynaud, C. et al. Mechanisms of pyrazinamide resistance in mycobacteria: importance of lack of uptake in addition to lack of pyrazinamidase activity. Microbiology 145, 1359–1367 (1999).
Nikonenko, B. V., Samala, R., Einck, L. & Nacy, C. A. Rapid, simple in vivo screen for new drugs active against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 48, 4550–4555 (2004).
Lei, B., Wei, C. J. & Tu, S. C. Action mechanism of antitubercular isoniazid. Activation by Mycobacterium tuberculosis KatG, isolation, and characterization of inha inhibitor. J. Biol. Chem. 275, 2520–2526 (2000).
Acknowledgements
We thank Dr Kenichiro Nagai, School of Pharmacy, Kitasato University, for the synthesis of calpinactam. This work was supported by JSPS KAKENHI Grant Numbers 16H05095 (to RU) and 21310146 (to HT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
We dedicate this work to Professor Satoshi Ōmura, a distinguished Nobel Prize awardee in Physiology or Medicine, 2015.
Rights and permissions
About this article
Cite this article
Yagi, A., Uchida, R., Hamamoto, H. et al. Anti-Mycobacterium activity of microbial peptides in a silkworm infection model with Mycobacterium smegmatis. J Antibiot 70, 685–690 (2017). https://doi.org/10.1038/ja.2017.23
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2017.23
This article is cited by
-
Alternatives to animal models to study bacterial infections
Folia Microbiologica (2023)
-
Inducible knockdown of Mycobacterium smegmatis MSMEG_2975 (glyoxalase II) affected bacterial growth, antibiotic susceptibility, biofilm, and transcriptome
Archives of Microbiology (2022)
-
Identification of structurally diverse menaquinone-binding antibiotics with in vivo activity against multidrug-resistant pathogens
Nature Microbiology (2021)
-
A novel diterpene agent isolated from Microbispora hainanensis strain CSR-4 and its in vitro and in silico inhibition effects on acetylcholine esterase enzyme
Scientific Reports (2020)
-
How to harness biosynthetic gene clusters of lasso peptides
Journal of Industrial Microbiology and Biotechnology (2020)