Abstract
Habitat fragmentation can change the ecological context of populations, rupturing genetic connectivity among them, changing genetic structure, and increasing the loss of genetic diversity. We analyzed mating system and pollen structure in two population fragments and two continuous forest populations of Dieffenbachia seguine (Araceae), an insect-pollinated understory herb in the tropical rain forest of Los Tuxtlas, México, using nine allozyme loci. Mating system analysis indicated almost complete outcrossing but some inbreeding among the adults. Pollen structure analysis indicated highly restricted pollen flow, both within and among populations. We showed that the effective pollination neighborhood was small in all populations, and slightly (though not significantly) smaller in fragments, partially as a consequence of an increase in density of reproductive individuals in those fragments. Using assignment analysis, we showed that all populations were strongly structured, suggesting that pollen and seed flow across the Los Tuxtlas landscape has been spatially restricted, though sufficient to maintain connectedness. Forest fragmentation at Los Tuxtlas has (so far) had limited impact on pollen dynamics, despite the changing ecological context, with reduced pollinator abundance being partially offset by increased flowering density in fragments. Continued outcrossing and limited pollen immigration, coupled with more extensive seed migration, should maintain genetic connectedness in D. seguine, if fragmentation is not further exacerbated by additional deforestation.
Similar content being viewed by others
Introduction
Pollen flow has an important influence on the evolutionary dynamics of plant populations. It has an important function in an individual's male fertility (Cunningham, 2000; Dick et al., 2003), as well as on the distribution of genetic variability within and among populations (Young et al., 1996; Sork et al., 1999). Forest fragmentation may create areas of unfavorable habitat and may erect ecological barriers for pollen vectors that reduce pollen flow, disrupting genetic connectivity among populations. Modification of pollen flow patterns can alter the amount and population structure of genetic variation (Young et al., 1996; Sork and Smouse, 2006), depending on the complex interactions of phenology, local density of conspecifics, and the degree to which pollen vectors overcome the effects of landscape change (Barrett and Harder, 1996; Smouse and Sork, 2004), and in some cases, impacting local adaptation.
Forest fragmentation also reduces population size, limits the local pool of potential mating partners (Young et al., 1996; Sork et al., 1999), and can sometimes alter the species composition and behavior of ensembles of pollen vectors (Kearns et al., 1998; Steffan-Dewenter and Tscharntke, 2002). Many insect pollinators are unable to fly through a matrix landscape, and others prefer particular sets of microenvironmental conditions, effectively breaking the connection between fragments, which become genetically independent units with small effective plant population sizes (Steffan-Dewenter and Tscharntke, 2002). In other cases, and for other insect-pollinated species, the matrix actually facilitates their movement (Dick et al., 2003; Sork et al., 2005), increasing long distance pollen flow and enhancing genetic panmixia over large areas. In addition, the distribution of genetic variation within and among population fragments will depend on the scale of the remnant habitat, relative to the area of the preexisting genetic neighborhoods. If fragmentation breaks up the breeding neighborhoods, decreasing genetic variation may be expected within fragments (Nason et al., 1997).
For conservation purposes, we need to determine whether habitat fragmentation affects connectivity among populations (Burczyk et al., 2004; O’Connell et al., 2006; Sork and Smouse, 2006), that is, whether population fragments have different pollination dynamics than do continuous populations (Smouse and Sork, 2004). Measurement of the extent of contemporary pollen flow enhances our understanding of the impact of landscape and ecological context on the factors that influence pollen dispersal on local populations and the scale over which they operate (Sork et al., 1999; Dyer and Sork, 2001; Burczyk et al., 2004).
The fragmented tropical forest in the Los Tuxtlas region of the Mexican Gulf provides a unique ecological arena, within which to study the process of gene flow via pollen movement and how that is impacted by environmental and ecological heterogeneity created by anthropogenic disturbance. The clonal herb Dieffenbachia seguine is an important component in the understory structure at Los Tuxtlas, occupying both large continuous forest and small disturbed fragments. Populations of D. seguine are large, but each population has only a few reproductive individuals at any one time. This species is pollinated by small beetles with limited tolerance of open conditions (Cuartas-Hernández, 2006), so the situation provides an interesting opportunity to study mating and pollen dispersal (Robledo-Arnuncio and Gil, 2005; Gonzales et al., 2006). We have reported earlier that natural populations of D. seguine at the Los Tuxtlas Reserve are genetically highly structured and diverse, but fragmented populations, while having higher population density, exhibit reduced arrays of multilocus genotypes (Cuartas-Hernández and Núñez-Farfán, 2006). Reduced seed production and high abortion rates for infructescences in population fragments suggest severe pollen limitation, relative to populations in the continuous forest (Cuartas-Hernández and Núñez-Farfán, unpublished). Numerically scarce pollinators (Cuartas-Hernández, 2006) and a dearth of multilocus genotypes in population fragments constitute a natural experimental system within which to investigate the effects of forest fragmentation on the patterns of pollen dispersal.
We assess the impacts of forest fragmentation on contemporary pollen dispersal in D. seguine, describing the patterns of pollen flow within and among populations, under contrasting fragmented and continuous forest conditions. We have three specific objectives: (1) to estimate mating system parameters and determine whether variation in these parameters is related to forest continuity; (2) to determine whether forest fragmentation increases pollen pool structure, describe the dynamics of pollen movement within populations, and provide estimates of the effective numbers of pollen parents and the effective sizes of pollination neighborhoods; and (3) to estimate genetic divergence among the four populations and determine the most likely population source of each male gamete, thus inferring the level of pollen immigration from neighboring populations. On the basis of genotypes of nine polymorphic allozyme loci, we quantified the proportion of matings (outcrossed, inbred, or selfed) that occur within populations; estimate the genetic heterogeneity of pollen pool sampled by different maternal parents, and estimated the proportion of immigrant pollen, providing information on the degree to which pollen is moving across the landscape.
Materials and methods
Study species
D. seguine (Araceae) is a clonally spreading perennial herb (ca. 80 cm in height) that inhabits the understory of primary tropical rain forest. It is distributed from southern Mexico to Guyana, Brazil, and Ecuador (Mayo et al., 1997). Populations are dense, although discretely and patchily distributed, with small numbers of reproductive individuals. The inflorescence consists of a spathe, with female flowers at the bottom and male flowers at the top; the species is protogynous. Inflorescences are visited by Dynastinae beetles from the genus Cyclocephala (Family Scarabaeidae) (Cuartas-Hernández, 2006). Infructescences consist of multiple small, round fruits with one seed each. The fruits are dispersed by birds of the genera Habia and Turdus (E Figueroa, personal communication). Each ramet bears a single open inflorescence at any one time, limiting the possibility of geitonogamous selfing. D. seguine is the only species from the genus in the region (Ibarra-Manríquez et al., 1997), where only a few populations remain in fragmented forest. The species is characteristic of primary tropical forest, but plants become chlorotic in highly disturbed sites, where their performance suffers (Personal Observations). The species has exacting requirements for pollinating beetles, whose survival in and ability to traverse inhospitable habitat are limited (Estrada et al., 1998).
Study site and sampling
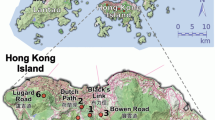
The study area is located at the Los Tuxtlas tropical forest Biosphere Reserve in the Mexican Gulf. The landscape of the Los Tuxtlas region is dominated by a network of tropical forest patches of different sizes, embedded in agricultural or grazing pasture habitats, resulting from three decades of deforestation. However, the Los Tuxtlas Natural Reserve (18°35′240 N, 95°04′629 W), administered by the Universidad Nacional Autonoma de Mexico, is a large area of continuous undisturbed forest. Knowledge concerning gene flow patterns of species in this region is relevant for conservation of the great diversity of the tropical forest in Los Tuxtlas region, which includes endemic, temperate, and tropical species (González - Soriano et al., 1997). D. seguine populations are facing a challenge to their persistence, due to the disruption of pollen dispersal and reduced fruit production. The undisturbed forest area at Los Tuxtlas is large, allowing the study of undisturbed pollination patterns and comparison of those with their counterparts in forest fragments. We established two plots in continuous forest (C1 and C2) at the Reserve (>640 ha) and two plots in nearby forest fragments (F1 in a 3 ha fragment and F2 in a 37 ha fragment). The two fragments correspond to those we have studied earlier, in which we detected significant reduction in both seed production and genotypic richness, relative to continuous forest, but which still possessed characteristics of primary tropical forest and supported large population sizes of D. seguine (Cuartas-Hernández, 2002). The populations are spatially isolated and are separated from each other by an average distance of 3.4 km (Figure 1), ranging from 1.75 (between C1 and C2) to 4.25 km (between F1 and F2).
We established a 20 × 50 m (0.1 ha) plot within each population in November 2002. Fruit production is sparse in D. seguine, so we marked and mapped each plant possessing a completely developed inflorescence within the plot (range 23–99 per plot). We collected leaf tissue from every flowering individual and stored the tissue at −70 °C for later identification of maternal genotype. After 5–6 months (April–May 2003), we collected all developed infructescences within the plot, before fruits were mature and exposed to bird dispersal, to sample all fruits within each infructescence. The number of sampled maternal families (mother and progeny) varied among populations, with a range of 11–24 (average=16), for a total of 65 families and 531 seedlings (Table 1). We hydrated all collected fruits for 24 h to facilitate peeling. We extracted and sterilized the seeds and sowed them in Petri dishes with agar, and then placed them in a germination chamber with controlled humidity, light, and temperature. After 2–3 months, we collected leaves from the seedlings and stored them at −70 °C, pending genetic analysis. We used starch gel electrophoresis and nine polymorphic allozyme loci to characterize genetic variation. Detailed electrophoretic assay methods, effective numbers of alleles, heterozygosity, and the number of different multilocus genotypes are presented in Cuartas-Hernández and Núñez-Farfán (2006).
Mating system analysis
The mixed mating system model assumes that a portion t of a seed family (or population) is comprised of outcrossed seeds, with s (=1–t) representing the proportion of selfed seeds. Some outcrossed matings can be with close relatives, and it is possible to estimate a biparental inbreeding rate as (tm−ts), where tm is a multilocus estimate of the outcrossing rate and ts is an average single-locus rate (Ritland and Jain, 1981). We used the MLTR program (version 3.0, Ritland, 2002) to obtain estimates of both tm and ts, assuming equal allele frequencies in the pollen and ovule pools, because there is no biological reason to presume the converse for a monoecious species. We also estimated Fp, the inbreeding coefficient of paternal parents and the correlation of paternity, rp = (2/1 + Fp), f, where f is the correlation of paternal gametes (Ritland, 1989), and where rp indicates the probability that two outcrossed seeds from a maternal family share the same paternal parent. We used an Expectation-Maximization algorithm to obtain maximum likelihood estimates, which is useful when some data are incomplete (Ritland, 2002). We estimated standard deviations and 95% confidence intervals with 1000 bootstrap trials, resampling entire families. We concluded that estimates of outcrossing rates, biparental inbreeding, and correlation of paternity were significantly different from each other when their 95% confidence intervals did not overlap, and significantly different from zero when the confidence intervals did not overlap with zero.
Pollen pool heterogeneity
Direct evaluation of current mating episodes can be accomplished with paternity analysis, but that approach requires virtually exhaustive sampling of reproductive adults over a considerable area and a high-resolution genetic marker battery to identify the precise individual that has contributed a particular male gamete to a seed (Marshall et al., 1998; Robledo-Arnuncio and Gil, 2005). Our allozyme assay battery is not sufficiently polymorphic to permit categorical paternal designation and our adult sampling, while complete within the plots, does not cover the entire portion of the Las Tuxtlas Reserve that might be relevant for pollination, so we have used an indirect method of pollen pool assessment instead (see review in Smouse and Sork, 2004).
We used TWOGENER, which assesses heterogeneity of the pollen pool sampled by different females within each population sample (Smouse et al., 2001), providing some information about the spatial distribution of mating pairs across the landscape (Austerlitz and Smouse, 2001a, 2001b, 2002). The null hypothesis is that maternal plants sample pollen randomly from a global (panmictic) pool. Using the genotypes of maternal plants and those of offspring sampled from them, male gametic genotypes were inferred for each seedling by substracting the maternal gametic contribution from the seedling (see Smouse et al., 2001). TWOGENER analysis calculates a genetic distance matrix between male gametes and executes an analysis of molecular variance to partition the male gametic variation into separate within- and among-mother components of variance (Excoffier et al., 1992), from which it computes an estimate of Φft, the fraction of the total male gametic variance that separates maternal sibships, which is essentially the correlation of paternal gametes, that is, Φft∼f in Ritland's (1989) formula. We conducted a separate one-level nested TWOGENER assessment for each population, using GENALEX 6 (Peakall and Smouse, 2006).
In populations with paternal inbreeding and with some fraction of self-fertilization, Φft must be adjusted for both (Austerlitz and Smouse, 2001b; Burczyk and Koralewski, 2005). We therefore adjusted the estimate of Φft for inbreeding in the adults (FP), computing Φ′ft=Φft/(1+FP)∼(1/2)·f, and for the selfing rate (s=1−t), computing Φ′′ft=(2·Φ′ft–s2)/2·(1−s)2, using the estimates of those parameters obtained from our MLTR analysis, which yields a final estimate of the effective number of pollen parents for the average maternal sibship of Nep=[2Φ′′ft]. We also estimated the effective pollination area as Aep=Nep/de, where de is the effective density of potential pollen donors (Smouse et al., 2001; Austerlitz and Smouse, 2001a). We have recorded density of flowering ramets in each population and assumed that the effective number is only 10% of the census number of flowering adults at any specific time (Gonzales et al., 2006). We used a t-test to compare the average Aep-values of population fragments (as a group) and continuous forest populations (as a group). The within group variances of Aep estimates were not identical for continuous forest and fragment groups, so we used Welch's approximate t-statistic to assess the significance of the difference, appropriate when samples have unequal variances (Zar, 1999).
Assignment analysis
We used assignment analysis as a means of estimating the level of genetic differentiation among adult plants and pollen populations and to infer possible inter-population dispersal events for both seeds and pollen. For the adult assignments, we included all reproductive adults from the four populations, and used a frequency-based assignment method (Paetkau et al., 1995). We also conducted an assignment analysis on male gametes sampled by the maternal parents. For this purpose, we used male gametic distance to assign individual male gametes to one of the four populations, using the squared distance from the i-th male gamete to the average male gamete extracted from the k-th population (k=C1, C2, F1, or F3),

where pij is the frequency of the j-th allele in the i-th individual (0, 1/2, 1) and p̄kj is the estimated allele frequency for the j-th allele in the k-th population. Squared distance was summed over all n alleles of the nine-locus genetic battery. The method is slightly biased in favor of assigning the gamete to its proper population, because the gamete in question contributes to the estimated mean for that population. To eliminate that bias, we adjusted the distance of a male gamete to its own population, multiplying di,i2 by [Ni/(Ni–1)]2. We calculated the genetic distance from each male gamete to each of the four populations, and then assigned that male gamete to whichever population had the minimum genetic distance, that is, to whichever population was most similar (Spielman and Smouse, 1976; Smouse et al., 1982).
For either analysis, the probability of correctly assigning an individual increases with the number of loci, the genetic separation of the populations under examination, and on the number of candidate populations (Smouse et al., 1982; Paetkau et al., 1995; Smouse and Chevillon, 1998). As D. seguine populations are highly structured, they constitute good candidates for this analysis (Smouse et al., 1982). The method is non-parametric and does not assume Hardy–Weinberg equilibrium, so it is more robust to failure of the H–W assumptions than are either the MLTR or the TWOGENER treatments. We calculated the fraction of male gamets or adult plant genotypes assigned to each of the studied populations.
Each assignment of an adult plant or pollen gamete to another population (cross-population assignment) conveys information about the level of genetic divergence among populations, and it is interpretable in terms of probabilities of propagule dispersal. If there is panmictic dispersal, there will be no meaningful divergence, and assignment rates to each of the four population samples from each of the population samples should be similar (∼1/4, by virtue of the fact that there are four populations). The greater the correct assignment rate (over 1/4), the greater the genetic divergence (Smouse et al., 1982; Smouse and Chevillon, 1998). The lower the rate of correct assignment rate, the higher is the implicit rate of propagule exchange.
Results
Mating system analysis
Our MLTR analyses showed that all populations were predominantly outcrossing, with multilocus outcrossing rates ranging from 0.82 to 1.00 (Table 1). Of the four populations, only F2 had a tm-estimate that departed significantly from 1.00, an indication of selfing. In self-incompatible plants like D. seguine, an apparent selfing rate of s=0.18 could reflect biparental inbreeding (Ritland and Jain, 1981). Alternatively, selfing is possible when pollinators are very scarce, even in plants that usually do not self-fertilize, and that has apparently occurred in the F2 fragment (Cuartas-Hernández, 2006). The average single-locus outcrossing rate, ts, ranged from 0.66 to 1.00. Estimates of biparental inbreeding ranged from −0.07 to 0.17, and the estimates for F2 and C2 were significantly greater than zero (0.15 and 0.17, respectively). Our correlated paternity estimates ranged from rp=0.14 to 0.99. In three populations, between 50 and 100% of offspring pairs within a family were full sibs. The mean value of rp=0.54, although this average is strongly affected by the value for population C1 (rp=0.14). This value indicates a small number of pollen donors contributing to each pollination visit, a common feature of insect-pollinated plants. The low rp estimate for C1 could be due to the inverse relationship with Fp (inbreeding in the parental population), which was much higher in C1 than in the other populations, explaining the high Nep estimate for this population (Table 2). Variation in the mating system parameters was large, but was not related to the fragmented or continuous condition of the forest.
Pollen pool heterogeneity
Our TWOGENER analyses indicated that maternal sibship structure of male gamete pools was pronounced for both fragmented and continuous populations, a result that is not in accord with the expectation of higher pollen structure in fragmented populations. The partition of gametic variance (Φft) within and among mothers was similar for all four populations (Table 2). The effect of the spatial genetic structure (asexual reproduction), Fp, and selfing (with asexually produced genet-mates), s, on the genetic structure of pollen was incorporated into Φ′ft and Φ′′ft, respectively, but Φft-values remain high, even after adjustment (Table 2). The estimated values of Nep from Φ′′ft ranged from 1.0 to 2.4 for the four populations, suggesting that the offspring from a single maternal sibship in D. seguine represent (at most) a small number of full sibships (Table 2). These value lie in the lower range of those for herbaceous species (Hardy et al., 2004). The estimated values of Nep, when considered jointly with low effective reproductive densities of pollen donors (Table 1) and a dearth of pollinators within any given population, indicates a very small effective pollination neighborhood size (Aep) in general, which although smaller for the fragments, is not significantly different (t=1.807, P=0.32) (Table 2).
Assignment analysis
On average, 65% of adult plants were assigned to their natal population (Table 3a), indicating low amounts of genetic overlap of those populations and a substantial level of genetic divergence among them. This pattern allows us to classify a genotype as (more or less) characteristic of its current population (Paetkau et al., 1995). The substantial divergence among adult populations should also be reflected in the pollen pools derived from them (Smouse et al., 1982), and male gamete assignments showed substantial genetic structure as well. On average, we assigned 71.5% of male gametes to the same population as the female they pollinated, indicating strong genetic divergence among local populations. Assignment of the remaining male gametes (28.5%) was variably distributed among the other three populations (Table 3b). Assignments to another population can be biased, of course, if the population of origin is not represented in the set of sampled populations, because the method will always designate a preferred population of origin (Cornuet et al., 1999), but clearly, any of these populations of adults could have served as a pollen source for the others (Tables 3a and b). In general, pollen immigration into populations was modest (on average, 31% into fragments and 26% into continuous forest plots), similar to observations from other species (Burczyk et al., 2004; Sork and Smouse, 2006). There was no detectable association between percentage of assigned male gametes and geographic distance between populations (results not shown) for either the fragmented or continuous populations. Viewed either from the perspective of pollen structure or from that of adult genotypic structure, the gene pools of D. seguine populations are highly differentiated on a relatively localized geographic scale, whether they are from fragmented or from continuous forest, suggesting low genetic connection among populations in the Los Tuxtlas region.
In summary, the results suggest that pollen movement is mostly confined to a single population, and the populations are noticeably differentiated, a result in keeping with the pronounced differentiation for adult plants detected in an earlier analysis (Cuartas-Hernández and Núñez-Farfán, 2006). Single-mother progenies appear to have been sired by no more than a few pollen donors and these patterns are consistent for both continuous and fragmented populations.
Discussion
Selfing and biparental inbreeding
In a companion experimental study, we used hand pollinations and found that 100% of self-pollinated inflorescences failed to produce fruits, clearly suggesting that D. seguine is strongly self-incompatible (Cuartas-Hernández and Nuñez-Farfán, in preparation). Here, however, mating system analyses indicate variation in outcrossing levels among populations, with F2 showing a departure of complete outcrossing due to self-fertilization or an elevated degree of biparental inbreeding (Ritland and Jain, 1981). More intense clonal propagation in F2 than in the other populations (Cuartas-Hernández, 2002) could increase the probability of mating among genetically identical ramets that, although spatially and physiologically separated, are capable of geitonogamous selfing. Pollen limitation due to scarcity of Cyclocephala beetles or very frequently, absence of pollinator visits in this fragment (Cuartas-Hernández and Núñez-Farfán, in preparation) could also drive plants to self-pollination, thus ensuring at least some seed production (Lloyd and Schoen, 1992), as suggested for D. seguine and several other aroid species in Guyana, where a capacity for self-pollination has been documented (Chouteau et al., 2008). Partial self-incompatibility has also been found for Dieffenbachia longispatha from Costa Rica (Young, 1986), implying some variation in mating system parameters within the genus, variation that becomes evident over geographic scales, possibly associated with disturbed habitats, as well as among species.
Biparental inbreeding significantly greater than zero was detected in populations C2 and F2, suggesting that crossing between genetically related plants occurs more frequently in these populations than expected from random mating (Ritland, 1984). Although it would be necessary to know the history of the populations to assign the causes of biparental inbreeding in these populations, it could result from spatial substructure of genotypes (Ennos and Clegg, 1982) and limited pollen flow (Sun and Ritland, 1998), both of which are plausibly attributable to vegetative propagation and short flight distance of pollinators.
Pollen vectors
Correlated paternity can arise from correlated dispersal events, which means that loads of pollen are deposited by single pollinators from the plant most recently visited (Sun and Ritland, 1998; Hardy et al., 2004). Codispersion of pollen from single donors is plausible because of (1) the tendency of pollinating beetles to remain feeding on a single inflorescence for as long as 2 days, presumably limiting the number of contributing pollen donors on a single visit, (2) the low number of simultaneously open inflorescences within populations, with different plants in different phenological stages (that is, non-receptive or female or male), and (3) very low pollinator abundance (Cuartas-Hernández, 2006).
Pollination neighborhoods
Effective pollination neighborhoods were small, implying that, in general, effective pollination distance is inherently small in these populations. Contemporary populations of D. seguine experiencing fragmentation exhibit enhanced inflorescence production, because of increases in light availability, thus increasing the density of reproductive individuals. At the same time, the abundance of scarabid beetles is positively associated with the area of the fragment at Los Tuxtlas, indicating a negative effect of fragmentation on these insects (Estrada et al., 1998; Cuartas-Hernández, 2006). As a consequence of altered ecology, the net effect is a slight (but non-significant) decrease in the effective pollination area, Aep, suggesting that increased flowering density can partially compensate for reduced pollinator abundance.
Connecting the populations
Genetic relationships estimated for the pool of male gametes within and among populations suggest that pollen pools are highly differentiated, in general, indicating that a high proportion of gametes come from local pollen donors. Long distance pollen movement should be infrequent in D. seguine, because the pollinating beetles are unable to fly through the matrix landscape and are very sensitive to desiccation in open areas (Estrada et al., 1998). The observed pattern in the assignment analysis for male gametes is congruent with that obtained for adult plants. It is possible that dispersal of seeds from one or a few plants intensifies genetic divergence among populations, because it is likely that birds consume numerous fruits from a single infructescence. Given the correlated paternity of the progeny from these fruits, seeds within an infructescence are collections of full-sib families. Some adult individuals were assigned to other populations, so non-trivial genetic affinity persists among populations. Over a few generations, there is enough movement of seeds (and even pollen) to connect these populations, which accounts for the non-trivial cross-population assignment (Paetkau et al., 1995; Waser and Strobeck, 1998).
The fact that the fraction of male gametes assigned to the local population was (on average) 8% higher than adult assignment rates suggests that pollen-mediated movement of genes has been more localized than the total movement of genes (pollen and seeds) between populations. Bird-mediated dispersal of seeds is the probable source of most contemporary immigration among populations, because they can move through riparian corridors and live fences (E Figueroa, personal communication), tending to attenuate genetic divergence among populations, despite the restricted pollen flow (insect mediated).
Conclusions and prognosis
The high genetic structure of both male gametes and adult plants in undisturbed forest and fragment populations reveal that populations of D. seguine are ecologically independent units, to a considerable extent, and that their internal genetic structures are probably the result of different historical trajectories. On the other hand, this study shows that forest fragmentation has (so far) not had a particularly negative impact on pollen-mediated gene flow in D. seguine populations. We provide empirical evidence of limited gene flow within and among populations, suggesting that this is the typical pattern for D. seguine and that small differences in pollen dynamics between populations could be a consequence of subtle differences in ecological characteristics. The scale of forest fragmentation at Los Tuxtlas has neither broken up the populations nor disrupted the mating neighborhoods of D. seguine, which has maintained its characteristic patterns of pollen flow in both fragmented and continuous forest. However, the mostly intra-patch scale on which it occurs enhances differentiation among populations. The strongly outcrossed mating system and eventual pollen flow can be expected to maintain genetic diversity and connectivity across the region. D. seguine is currently holding its own, both demographically and genetically, in the still changing ecological context of Los Tuxtlas.
How D. seguine will fare in the future will depend on a variety of factors. We have quantified pollen flow for a single reproductive event (season), and it remains possible that in subsequent years, the situation will change, due to ongoing changes of the ecosystem. Temporal replication in other systems has shown that mating system may change in response to environmental conditions (Hoebee et al., 2007). In other cases, year-to-year variation in mating system have not been detected (Irwin et al., 2003). Quite apart from the challenge of monitoring pollen flow patterns over several reproductive events, there is a need to assess germination success and seedling performance of natural progenies, if we are to achieve a fuller understanding of the long-term adaptive consequences of fragmentation.
References
Austerlitz F, Smouse PE (2001a). Two-generation analysis of pollen flow across a landscape. II. Relation between ΦFT, pollen dispersal and interfemale distance. Genetics 157: 851–857.
Austerlitz F, Smouse PE (2001b). Two-generation analysis of pollen flow across landscape. III. Impact of within population structure. Genet Res 78: 271–278.
Austerlitz F, Smouse PE (2002). Two-generation analysis of pollen flow across a landscape. IV. Estimating the dispersal parameter. Genetics 161: 355–363.
Barrett SCH, Harder LD (1996). Ecology and evolution of plant mating. Trends Ecol Evol 2: 73–79.
Burczyk J, DiFazzio SP, Adams WT (2004). Gene flow in forest trees: how far do genes really travel? For Genet 11: 1–14.
Burczyk J, Koralewski TE (2005). Parentage versus two-generation analyses for estimating pollen-mediated gene flow in plant populations. Mol Ecol 14: 2525–2537.
Chouteau M, Gibernau M, Barabé D (2008). Relationships between floral characters, pollination mechanisms, life forms, and habitats in Araceae. Bot J Linn Soc 156: 29–42.
Cornuet JM, Piry S, Luikart G, Estoup A, Solignac M (1999). New methods employing multilocus genotypes to select or exclude populations as origins of individuals. Genetics 153: 1989–2000.
Cuartas-Hernández S (2002). Efectos ecológicos y genéticos de la fragmentación en las poblaciones de Dieffenbachia seguine (Araceae) en Los Tuxtlas, Veracruz, México. MSc thesis, Universidad Nacional Autónoma de México, Mexico.
Cuartas-Hernández S (2006). Efectos de la fragmentación del bosque tropical: biología reproductiva y sistema de apareamiento en poblaciones de Dieffenbachia seguine (Araceae). PhD thesis, Universidad Nacional Autónoma de México, Mexico.
Cuartas-Hernández S, Núñez-Farfán J (2006). The genetic structure of the tropical understory herb Dieffenbachia seguine L. before and after forest fragmentation. Evol Ecol Res 8: 1061–1075.
Cunningham SA (2000). Effects of habitat fragmentation on the reproductive ecology of four plant species in Mallee Woodland. Conserv Biol 14: 758–768.
Dick CW, Etchelecu G, Austerlitz F (2003). Pollen dispersal of tropical trees (Dinizia excelsa: Fabaceae) by native insects and African honrybees in pristine and fragmented Amazonian forest. Mol Ecol 12: 753–764.
Dyer RJ, Sork VL (2001). Pollen pool heterogeneity in shortleaf pine, Pinus equinata Mill. Mol Ecol 10: 859–866.
Ennos RA, Clegg MT (1982). Effect of population substructuring on estimates of outcrossing rate of grain sorghum (Sorghum bicolor). Theor Appl Gen 66: 323–327.
Estrada A, Coates-Estrada R, Anzures-Dadda A, Cammarano P (1998). Dung and carrion beetles in tropical rain forest fragments and agricultural habitats at Los Tuxtlas, Mexico. J Trop Ecol 14: 577–593.
Excoffier L, Smouse PE, Quattro JM (1992). Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction sites. Genetics 131: 479–491.
Gonzales E, Hamrick JL, Smouse PE, Dyers RJ (2006). Pollen-mediated gene dispersal within continuous and fragmented populations of forest understorey species, Trillium cuneatum. Mol Ecol 15: 2047–2058.
González-Soriano E, Dirzo R, Vogt RC (1997). Historia Natural de Los Tuxtlas. Universidad Nacional Autónoma de México: México D.F.
Hardy OJ, Gonzáles-Martínez SC, Colas B, Fréville H, Mignot A, Olivieri I (2004). Fine-scale genetic structure and gene dispersal in Centaurea corymbosa (Asteraceae). Genetics 168: 1601–1614.
Hoebee SE, Arnold U, Düggelin C, Gugerli F, Brodbeck S, Rotach P et al. (2007). Mating patterns and contemporary gene flow by pollen in a large continuous and a small isolated population of the scattered forest tree Sorbus torminalis. Heredity 99: 47–55.
Irwin AJ, Hamrick JL, Godt MJW, Smouse PE (2003). A multiyear estimate of the effective pollen donor pool for Albizia julibrissin. Heredity 90: 187–194.
Ibarra-Manríquez G, Martínez-Ramos M, Dirzo R, Núñez-Farfán J (1997). La Vegetación. In: González-Soriano E, Dirzo R, Vogt RC (eds)Historia Natural de Los Tuxtlas. Universidad Nacional Autónoma de México: Mexico D.F., pp 61–86.
Kearns CA, Intuye DW, Waser NM (1998). Endangered mutualisms: the conservation of plant-pollinator interactions. Ann Rev Ecol Syst 29: 83–112.
Lloyd DG, Schoen DJ (1992). Self- and cross-fertilization in plants. I. Functional dimensions. Int J Plant Sci 153: 358–369.
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998). Statistical confidence for likelihood based paternity inference in natural populations. Mol Ecol 7: 639–655.
Mayo SJ, Bogner J, Boyce PC (1997). The Genera of Araceae. Continental Printing: Belgium.
Nason JD, Aldrich PR, Hamrick JL (1997). Dispersal and the dynamics of genetic structure in fragmented tropical tree populations. In: Laurance WF, Bierregaard RO (eds). Tropical Forest Remnants: Ecology, Management, and Conservation of Fragmented Communities. University of Chicago Press: Chicago, pp 304–320.
O’Connell LM, Mosseller A, Rajora OP (2006). Impacts of forest fragmentation on the mating system and genetic diversity of white spruce (Picea glauca) at the landscape level. Heredity 97: 418–426.
Paetkau D, Calvert W, Stirling I, Strobeck C (1995). Microsatellite analysis of population structure in Canadian polar bears. Mol Ecol 4: 347–354.
Peakall R, Smouse PE (2006). GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6: 288–295.
Ritland K, Jain SK (1981). A model for the estimation of outcrossing rate and gene frequencies using n independent loci. Heredity 47: 35–52.
Ritland K (1984). The effective proportion of self-fertilization with consanguineous matings in inbred populations. Evolution 43: 848–859.
Ritland K (1989). Correlated matings in the partial selfer, Mimulus guttatus. Evolution 43: 848–859.
Ritland K (2002). Extensions of models for the estimation of mating systems using n independent loci. Heredity 88: 221–228.
Robledo-Arnuncio JJ, Gil L (2005). Patterns of pollen dispersal in a small population of Pinus sylvestris L. revealed by total-exclusion paternity analysis. Heredity 94: 13–22.
Smouse PE, Sork VL (2004). Measuring pollen flow in forest trees: an exposition of alternative approaches. Forest Ecol Manage 197: 21–38.
Smouse PE, Dyer RJ, Westfall RD, Sork VL (2001). Two-generation analysis of pollen flow across a landscape. I. Male gamete heterogeneity among females. Evolution 55: 260–271.
Smouse PE, Chevillon C (1998). Analytical aspects of population-specific DNA-fingerprinting for individuals. J Hered 89: 143–150.
Smouse PE, Spielman RS, Park MH (1982). Multiple-locus allocation of individual groups as a function of the genetic variation within and differences among human populations. Am Nat 119: 445–463.
Sork VL, Smouse PE (2006). Genetic analysis of landscape connectivity in tree populations. Landscape Ecol 21: 821–836.
Sork VL, Smouse PE, Apsit VJ, Dyer RJ, Westfall RD (2005). A two-generation analysis of pollen pool genetic structure in flowering dogwood, Cornus florida (Cornaceae), in the Missouri Ozarks. Am J Bot 92: 262–271.
Sork VL, Nason J, Campbell DR, Fernandez JF (1999). Landscape approaches to historical and contemporary gene flow in plants. Trends Ecol Evol 14: 219–224.
Spielman RS, Smouse PE (1976). Multivariate classification of human populations. I. Allocation of Yanomama Indians to villages. Am J Hum Genet 28: 317–331.
Steffan-Dewenter I, Tscharntke T (2002). Insect communities and biotic interactions on fragmented calcareous grasslands-a mini review. Biol Conserv 104: 275–284.
Sun M, Ritland K (1998). Mating system of yellow starthistle (Centurea solstitialis), a successful colonizer in North America. Heredity 80: 225–232.
Waser PM, Strobeck C (1998). Genetic signatures of interpopulation dispersal. Trends Ecol Evol 13: 43–44.
Young A, Boyle T, Brown T (1996). The population genetic consequences of habitat fragmentation for plants. Trends Ecol Evol 11: 413–418.
Young H (1986). Beetle pollination in Dieffenbachia longispatha (Araceae). Am J Bot 73: 931–944.
Zar JH (1999). Bioestatistical Analysis Fourth edition Prentice Hall: Upper Saddle River, NJ.
Acknowledgements
We thank J Vargas for assistance with isozyme electrophoresis, B Sinaca for field assistance, P Sosenski for the collection of seedlings tissue, and EA Gonzales and JJ Robledo-Arnuncio for the advice on the use of statistical software. We also thank the personnel of the Los Tuxtlas Biological Station (UNAM) for permission to use the facilities during the study, as well as the members of Laboratorio de Genética Ecológica y Evolucion for logistic support. We also thank a trio of anonymous reviewers for voluminous helpful critique. SC-H was supported by a Fellowship from the Posgrado en Ciencias Universidad Nacional Autónoma de México. PES was supported by NJAES/USDA-17111, NSF-DEB-0211430, and NSF-DEB-0514956. The research itself was funded by Semarnat—ConacyT 0355/Q and PAPPIT IN224908 grants to JN-F.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cuartas-Hernández, S., Núñez-Farfán, J. & Smouse, P. Restricted pollen flow of Dieffenbachia seguine populations in fragmented and continuous tropical forest. Heredity 105, 197–204 (2010). https://doi.org/10.1038/hdy.2009.179
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hdy.2009.179
Keywords
This article is cited by
-
Poisonous plants of Belize: a mini toxicological review
Advances in Traditional Medicine (2022)
-
Frugivore-Mediated Selection in A Habitat Transformation Scenario
Scientific Reports (2017)
-
Population at the edge: increased divergence but not inbreeding towards northern range limit in Acer campestre
Tree Genetics & Genomes (2014)