Abstract
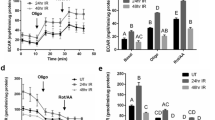
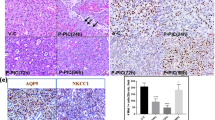
Head and neck irradiation (IR) during cancer treatment causes by-stander effects on the salivary glands leading to irreversible loss of saliva secretion. The mechanism underlying loss of fluid secretion is not understood and no adequate therapy is currently available. Delivery of an adenoviral vector encoding human aquaporin-1 (hAQP1) into the salivary glands of human subjects and animal models with radiation-induced salivary hypofunction leads to significant recovery of saliva secretion and symptomatic relief in subjects. To elucidate the mechanism underlying loss of salivary secretion and the basis for AdhAQP1-dependent recovery of salivary gland function we assessed submandibular gland function in control mice and mice 2 and 8 months after treatment with a single 15-Gy dose of IR (delivered to the salivary gland region). Salivary secretion and neurotransmitter-stimulated changes in acinar cell volume, an in vitro read-out for fluid secretion, were monitored. Consistent with the sustained 60% loss of fluid secretion following IR, a carbachol (CCh)-induced decrease in acinar cell volume from the glands of mice post IR was transient and attenuated as compared with that in cells from non-IR age-matched mice. The hAQP1 expression in non-IR mice induced no significant effect on salivary fluid secretion or CCh-stimulated cell volume changes, except in acinar cells from 8-month group where the initial rate of cell shrinkage was increased. Importantly, the expression of hAQP1 in the glands of mice post IR induced recovery of salivary fluid secretion and a volume decrease in acinar cells to levels similar to those in cells from non-IR mice. The initial rates of CCh-stimulated cell volume reduction in acinar cells from hAQP1-expressing glands post IR were similar to those from control cells. Altogether, the data suggest that expression of hAQP1 increases the water permeability of acinar cells, which underlies the recovery of fluid secretion in the salivary glands functionally compromised post IR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Siegel R, Ma J, Zou Z, Jemal A . Cancer statistics. CA Cancer J Clin 2014; 64: 9–29.
Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer 2010; 18: 1039–1060.
Vissink A, Mitchell JB, Baum BJ, Limesand KH, Jensen SB, Fox PC et al. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys 2010; 78: 983–991.
Amerongen AV, Veerman EC . Saliva—the defender of the oral cavity. Oral Dis 2002; 8: 12–22.
Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer 2010; 18: 1061–1079.
Liu X, Cotrim A, Teos L, Zheng C, Swaim W, Mitchell J et al. Loss of TRPM2 function protects against irradiation-induced salivary gland dysfunction. Nat Commun 2013; 4: 1515.
Spiegelberg L, Braks JA, Djasim UM, Farrell E, van der Wal KG, Wolvius EB . Effects of hyperbaric oxygen therapy on the viability of irradiated soft head and neck tissues in mice. Oral Dis 2014; 20: e111–e119.
Takahashi A, Inoue H, Mishima K, Ide F, Nakayama R, Hasaka A et al. Evaluation of the effects of quercetin on damaged salivary secretion. PLoS One 2015; 10: e0116008.
Takeda I, Kizu Y, Yoshitaka O, Saito I, Yamane GY . Possible role of nitric oxide in radiation-induced salivary gland dysfunction. Radiat Res 2003; 159: 465–470.
Vitolo JM, Cotrim AP, Sowers AL, Russo A, Wellner RB, Pillemer SR et al. The stable nitroxide tempol facilitates salivary gland protection during head and neck irradiation in a mouse model. Clin Cancer Res 2004; 10: 1807–1812.
Xu L, Yang X, Cai J, Ma J, Cheng H, Zhao K et al. Resveratrol attenuates radiation-induced salivary gland dysfunction in mice. Laryngoscope 2013; 123: E23–E29.
Nanduri LS, Maimets M, Pringle SA, van der Zwaag M, van Os RP, Coppes RP . Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol 2011; 99: 367–372.
Cotrim AP, Sowers A, Mitchell JB, Baum BJ . Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol Ther 2007; 15: 2101–2106.
Knox SM, Lombaert IM, Haddox CL, Abrams SR, Cotrim A, Wilson AJ et al. Parasympathetic stimulation improves epithelial organ regeneration. Nat Commun 2013; 4: 1494.
Cotrim AP, Yoshikawa M, Sunshine AN, Zheng C, Sowers AL, Thetford AD et al. Pharmacological protection from radiation +/− cisplatin-induced oral mucositis. Int J Radiat Oncol 2012; 83: 1284–1290.
Epperly MW, Wegner R, Kanai AJ, Kagan V, Greenberger EE, Nie S et al. Effects of MnSOD-plasmid liposome gene therapy on antioxidant levels in irradiated murine oral cavity orthotopic tumors. Radiat Res 2007; 167: 289–297.
Guo H, Seixas-Silva JA Jr, Epperly MW, Gretton JE, Shin DM, Bar-Sagi D et al. Prevention of radiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (SOD2) transgene. Radiat Res 2003; 159: 361–370.
van Luijk P, Pringle S, Deasy JO, Moiseenko VV, Faber H, Hovan A et al. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci Transl Med 2015; 7: 305ra147.
Timiri Shanmugam PS, Dayton RD, Palaniyandi S, Abreo F, Caldito G, Klein RL et al. Recombinant AAV9-TLK1B administration ameliorates fractionated radiation-induced xerostomia. Hum Gene Ther 2013; 24: 604–612.
Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One 2008; 3: e2063.
Nanduri LS, Lombaert IM, van der Zwaag M, Faber H, Brunsting JF, van Os RP et al. Salisphere derived c-Kit+ cell transplantation restores tissue homeostasis in irradiated salivary gland. Radiother Oncol 2013; 108: 458–463.
Baum BJ, Wellner RB, Zheng C . Gene transfer to salivary glands. Int Rev Cytol 2002; 213: 93–146.
Delporte C, O'Connell BC, He X, Lancaster HE, O'Connell AC, Agre P et al. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci USA 1997; 94: 3268–3273.
Shan Z, Li J, Zheng C, Liu X, Fan Z, Zhang C et al. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol Ther 2005; 11: 444–451.
Baum BJ, Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc Natl Acad Sci USA 2012; 109: 19403–19407.
Melvin JE, Yule D, Shuttleworth T, Begenisich T . Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 2005; 67: 445–469.
Ambudkar IS . Calcium signalling in salivary gland physiology and dysfunction. J Physiol 2015.
O'Connell AC, Redman RS, Evans RL, Ambudkar IS . Radiation-induced progressive decrease in fluid secretion in rat submandibular glands is related to decreased acinar volume and not impaired calcium signaling. Radiat Res 1999; 151: 150–158.
Teos LY, Zhang Y, Cotrim AP, Swaim W, Won JH, Ambrus J et al. IP3R deficit underlies loss of salivary fluid secretion in Sjogren's Syndrome. Sci Rep 2015; 5: 13953.
Delporte C, Hoque AT, Kulakusky JA, Braddon VR, Goldsmith CM, Wellner RB et al. Relationship between adenovirus-mediated aquaporin 1 expression and fluid movement across epithelial cells. Biochem Biophys Res Commun 1998; 246: 584–588.
Li J, Nielsen S, Dai Y, Lazowski KW, Christensen EI, Tabak LA et al. Examination of rat salivary glands for the presence of the aquaporin CHIP. Pflugers Arch 1994; 428: 455–460.
Zheng C, Baum BJ, Liu X, Goldsmith CM, Perez P, Jang SI et al. Persistence of hAQP1 expression in human salivary gland cells following AdhAQP1 transduction is associated with a lack of methylation of hCMV promoter. Gene Ther 2015; 22: 758–766.
Zheng CY, Baum BJ . Evaluation of viral and mammalian promoters for use in gene delivery to salivary glands. Mol Ther 2005; 12: 528–536.
Cotrim AP, Hyodo F, Matsumoto K, Sowers AL, Cook JA, Baum BJ et al. Differential radiation protection of salivary glands versus tumor by Tempol with accompanying tissue assessment of Tempol by magnetic resonance imaging. Clin Cancer Res 2007; 13: 4928–4933.
Zheng C, Cotrim AP, Rowzee A, Swaim W, Sowers A, Mitchell JB et al. Prevention of radiation-induced salivary hypofunction following hKGF gene delivery to murine submandibular glands. Clin Cancer Res 2011; 17: 2842–2851.
Brown AM, Rusnock EJ, Sciubba JJ, Baum BJ . Establishment and characterization of an epithelial cell line from the rat submandibular gland. J Oral Pathol Med 1989; 18: 206–213.
Liu X, Bandyopadhyay BC, Nakamoto T, Singh B, Liedtke W, Melvin JE et al. A role for AQP5 in activation of TRPV4 by hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J Biol Chem 2006; 281: 15485–15495.
Acknowledgements
We thank Dr James B Mitchell, Chief of the Radiation Oncology Branch in NCI, NIH, for his longstanding collaboration with our group, as well as for allowing us to use their IR facilities for these studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Teos, L., Zheng, CY., Liu, X. et al. Adenovirus-mediated hAQP1 expression in irradiated mouse salivary glands causes recovery of saliva secretion by enhancing acinar cell volume decrease. Gene Ther 23, 572–579 (2016). https://doi.org/10.1038/gt.2016.29
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2016.29
This article is cited by
-
Mitochondria-targeted antioxidant protects against irradiation-induced salivary gland hypofunction
Scientific Reports (2021)
-
Generation of orthotopically functional salivary gland from embryonic stem cells
Nature Communications (2018)
-
Late responses to adenoviral-mediated transfer of the aquaporin-1 gene for radiation-induced salivary hypofunction
Gene Therapy (2017)